Summary
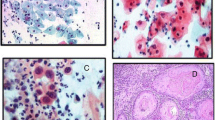
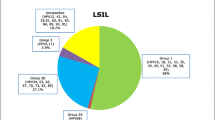
By means of a consensus polymerase chain reaction (PCR) method, the prevalence of HPV types was determined in cervical biopsies from 137 women referred to the gynecological outpatient clinic for colposcopy because of an abnormal cervical smear. The prevalence of HPV was 80.3%. There was a statistically highly significant rise in the prevalence of the oncogenic HPV types (16, 18, 31, 33) with increasing severity of cervical intraepithelial neoplasia (CIN I to III), indicating a role for these HPV types in the pathogenesis of cervical cancer. The prevalence of other HPV types decreased significantly with the severity of the lesion, suggesting that these HPV types play a less significant role in this process. These data indicate that HPV typing with PCR may be a valuable tool for distinguishing between highrisk and low-risk cervical lesions. Furthermore, our results suggest that the detection of HPV types by consensus PCR in the cervix of patients with an abnormal smear but without histologically detectable CIN is a useful tool for predicting which of these patiens will eventually develop CIN. Finally, a relatively low percentage (3%) of HPV double infections is reported in this study.
Similar content being viewed by others
References
Arends MJ, Donaldson YK, Duvall E, Wyllie AH, Bird CC (1991) HPV in full thickness cervical biopsies: high prevalence in CIN 2 and CIN 3 detected by a sensitive PCR method. J Pathol 165:301–309
Bauer HM, Yi Ting MS, Greer CE, Chambers JC, Tashiro CJ, Chimera J, Reingold A, Manos MM (1991) Genital human papillomavirus infection in female university students as determined by a PCR-based method. JAMA 265:472–477
Brandsma J, Burk R, Lancaster W, Pfister H, Schiffman MH (1989) Interlaboratory variation as an explanation for varying prevalence estimates of human papillomavirus infection. Int J Cancer 43:260–262
Brule AJC van den, Snijders PJF, Gordijn RLJ, Bleker OP, Meijer CJLM, Walboomers JMM (1990) General primer mediated polymerase chain reaction permits the detection of sequenced and still unsequenced human papillomavirus genotypes in cervical scrapes and carcinomas. Int J Cancer 45:644–649
Brule AJC van den, Walboomers JMM, Du Maine M, Kenemans P, Meijer CJLM (1991) Difference in prevalence of human papillomavirus genotypes in cytomorphologically normal cervical smears is associated with a history of cervical intraepithelial neoplasia. Int J Cancer 48:404–408
Campion MJ, McCance DJ, Cuzick J, Singer A (1986) Progressive potential of mild cervical atypia: prospective cytological, colposcopic, and virological study. The Lancet ii: 237–240
Cornelissen MTE, van den Tweel JG, Struyk APHB, Jebbink MF, Briët M, van der Noordaa J, ter Schegget J (1989) Localization of human papillomavirus type 16 DNA using the polymerase chain reaction in the cervix uteri of women with cervical intraepithelial neoplasia. J Gen Virol 70:2555–2562
Cornelissen MTE, van den Tweel JG, Struyk APHB, Briel MA, Smit HL, van der Noordaa J, ter Schegget J (1990) Distribution of HPV16 DNA in the cervix uteri containing cervical dysplasia. In: Howley PM, Broker TR (eds) Papillomaviruses. UCLA Symp Mol Cell Biol 124: Wiley-Liss, New York, pp 25–32
Crook T, Wrede D, Vousden KH (1991) p53 Mutation in HPV negative human cervical carcinoma cell lines. Oncogene 6:873–875
Crum CP, Mitao M, Levine RU, Silverstein S (1985) Cervical papillomaviruses segregate within morphologically distinct precancerous lesions. J Virol 54:675–681
Fujinaga Y, Shimada M, Okazawa K, Fukushima M, Kato I, Fujinaga K (1991) Simultaneous detection and typing of genital human papillomavirus DNA using the polymerase chain reaction. J Gen Virol 72:1039–1044
Greer CE, Peterson SL, Kiviat NB, Manos MM (1991) PCR amplification from paraffin-embedded tissues: effects of fixative and fixation time. Am J Clin Pathol 95:117–124
Kiyabu MT, Shibata D, Arnheim N, Martin WJ, Fitzgibbons PL (1989) Detection of human papillomavirus in formalin-fixed, invasive squamous carcinomas using the polymerase chain reaction. Am J Surg Pathol 13:221–224
Koutsky L (1991) Role of epidemiology in defining events that influence transmission and natural history of anogenital papillomavirus infections. J Nat Cancer Inst 83:978–979
Koutsky LA, Galloway DA, Holmes KK (1988) Epidemiology of genital human papillomavirus infection. Epidemiologic Rev 10:122–163
Ley C, Bauer HM, Reingold A, Schiffman MH, Chambers JC, Tashiro CJ, Manos MM (1991) Determinants of genital human papillomavirus infection in young women. J Natl Cancer Inst: 997–1003
Manos MM, Ting Y, Wright DK, Lewis AJ, Broker TR, Wolinsky SM (1989) The use of polymerase chain reaction amplification for the detection of genital human papillomaviruses. Cancer Cells 7:209–214
McCance DJ, Kopan R, Fuchs E, Laimins LA (1988) Human papillomavirus type 16 alters human epithelial cell differentiation in vitro. Proc Natl Acad Sci USA 85:7169–17173
Munoz N, Bosch X, Kaldor JM (1988) Does human papillomavirus cause cervical cancer? The state of the epidemiological evidence. Br J Cancer 57:1–5
Nuovo GJ (1990) Human papillomavirus DNA in genital tract lesions histologically negative for condylomata; analysis by in situ, Southern blot hybridisation and the polymerase chain reaction. Am J Surg Pathol 14:643–651
Nuovo GJ, Darfler Mm, Impraim CC, Bromley SE (1991) Occurrence of multiple types of human papillomavirus in genital tract lesions. Am J Pathol 138:53–58
Reid R, Greenberg M, Jenson AB, Husain M, Willett J, Daoud Y, Temple G, Stanhope CR, Sherman AI, Phipps GD, Lorincz AT (1987) Sexually transmitted papillomaviral infections. Am J Obstet Gynecol 156:212–222
Resnick RM, Cornelissen MTE, Wright DK, Eichinger GH, Fox HS, ter Schegget J, Manos MM (1990) Detection and typing of HPV in archival cervical cancer specimens using DNA amplification consensus primers. J Nat Cancer Inst 82:1477–1484
Riou G, Favre M, Jeannel D, Bourhis J, le Doussal V, Orth G (1990) Association between poor prognosis in early-stage invasive cervival carcinomas and non-detection of HPV DNA. Lancet 335:1171–1174
Roman A, Five KH (1989) Human papillomaviruses: Are we ready to type? Clin Microbiol Rev 2:166–190
Schneider A, Kraus H, Schuhmann R, Gissmann L (1985) Papillomavirus infection of the lower genital tract: Detection of viral DNA in gynecological swabs. Int J Cancer 35:443–448
Syrjänen K, Syrjänen S (1989) Concept of the existence of human papillomavirus (HPV) DNA in histologically normal squamous epithelium of the genital tract should be re-evaluated. Acta Obstet Gynecol Scand 68:613–617
Tham KM, Chow VTK, Singh P, Tock EPC, Chng KC, LimTan SK, Sng ITY, Bernard HU (1991) Diagnostic sensitivity of polymerase chain reaction and Southern blot hybridisation for the detection of human papillomavirus DNA in biopsy specimens from cervical lesions. Am J Clin Pathol 95:638–646
Twiggs LB, Okagaki T, Clark B, Fukushima M, Ostrow R, Faras A (1988) A clinical, histopathologic, and molecular biologic investigation of vulva intraepithelial neoplasia. Int J Gynecol Pathol 7:48–55
Vermund SH, Schiffman MH, Goldberg GH, Ritter DB, Weltman A, Burk RD (1989) Molecular diagnosis of genital human papillomavirus infection: comparison of two methods used to collect exfoliated cervical cells in the genital tract. Am J Obstet Gynecol 160:304–308
Vousden KH (1989) Human papillomaviruses and cervical carcinoma. Cancer Cells 1:43–49
Wilczynski SP, Pearlman L, Walker J (1988) Identification of HPV 16 early genes retained in cervical carcinomas. Virology 166:624–627
Yoshikawa H, Kawana T, Mizuno M, Yoshikura H, Iwamoto A (1991) Detection and typing of multiple genital human papillomaviruses by DNA amplification with consensus primers. Jpn J Cancer Res 2:524–531
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cornelissen, M.T.E., Bots, T., Briët, M.A. et al. Detection of human papillomavirus types by the polymerase chain reaction and the differentiation between high-risk and low-risk cervical lesions. Virchows Archiv B Cell Pathol 62, 167–171 (1992). https://doi.org/10.1007/BF02899679
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02899679