Abstract
Objectives
The present study investigated the involvement of oxidative stress in the degeneration of the cerebellum during methylmercury (MeHg) intoxication and the protective effect of α-tocopherol (Vit E) against MeHg toxicity.
Methods
After 5 mg/kg of MeHg was administered to Wistar rats for 12 consecutive days, the cerebellum were examined histopathologically. In addition, the same amount of MeHg was administered to 3 different groups of Wistar rats: rats with a Vit E-deficient diet, rats fed 150 mg/kg of Vit E for 20 consecutive days after initial MeHg administration, and rats with an ordinary diet.
Results
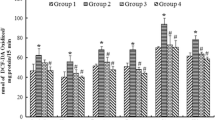
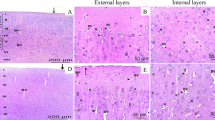
Positive immunoreactivity against anti-hydroxynonenal (HNE), a marker of lipid peroxidation, was observed in the cerebellum after MeHg administration. Levels of thiobarbituric acid reactive substance (TBARS), another marker of lipid peroxidation, and those of protein carbonyl, a biomarker for protein oxidation, increased after MeHg administration. In the rats with MeHg and a Vit E-deficient diet, mortality and prevalence of piloerection significantly increased, and in the rats with MeHg and Vit E, mortality, piloerection, retracted and crossed hind leg, and ataxic gait significantly decreased, compared with the rats with MeHg alone. The levels of NO2 − and NO3 − in the serum significantly increased in the rats with MeHg alone 14 days after the initial MeHg administration, but were significantly suppressed by Vit E administration.
Conclusions
Oxidative stress, especially lipid peroxidation, may play an important role in the cerebellar degeneration process during MeHg intoxication and Vit E may play a protective role against MeHg toxicity as an effective antioxidant.
Similar content being viewed by others
References
Hunter D Bomford RR, Russell DS. Poisoning by methyl mercury compounds. Quart. J. Med. 1940; 9: 193–213.
Hunter D, Russell DS. Focal cerebral and cerebellar atrophy in a human subject due to orgnanic mercury compounds. J. Neurol. Neurosurg. Psychiat. 1954; 17: 235–241.
Takeuchi T. Pathology of Minamata disease. In Study Group of Minamata disease, Kumamoto University (ed. Kutsuna, M.) Shuhan Publisher, Tokyo, Japan. 1968. pp. 141–228.
Takeuchi T. Pathology of Minamata disease. With special reference to its pathogenesis. Acta. Pathol. Jpn., 1982; 32: Suppl 1: 73–99.
Nagashima KA. Review of experimental methylmercury toxicity in rats: neuropathology and evidence for apoptosis. Toxicol. Pathol. 1997; 25: 624–631.
Rosen DR. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 1993; 364: 362.
Yamashita T, Ando Y, Obayashi K, Terazaki H, Sakashita N, Uchida K, Ohama E, Ando M, Uchino M. Oxidative injury is present in Purkinje cells in patients with olivopontocerebellar atrophy. J. Neurol. Sci. 2000; 175: 107–110.
Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990; 87: 1620–1624.
Yamashita T, Ando Y, Sakashita N, Hirayama K, Tanaka Y, Tashima K, Uchino M, Ando M. Role of nitric oxide in the cerebellar degeneration during methylmercury intoxication. Biochim. Biophys. Acta. 1997; 1334: 303–311.
Ikeda M, Komachi H, Sato I, Himi T, Yuasa T, Murota S. Induction of neuronal nitric oxide synthase by methylmercury in the cerebellum. J. Neurosci. Res. 1999; 55: 352–356.
Shinyashiki M, Kumagai Y, Nakajima H, Nagafune J, Homma-Takeda S, Sagai M, Shimojo, N. Differential changes in rat brain nitric oxide synthase in vivo and in vitro by methylmercury. Brain. Res. 1998; 798: 147–155.
Miura N, Kaneko S, Hosoya S, Furuchi T, Miura K, Kuge S, Naganuma A. Overexpression of L-glutamine: D-fructose-6-phosphate amidotransferase provides resistance to methylmercury in Saccharomyces cerevisiae. FEBS Lett. 1999; 458: 215–218.
Lucesoli F. Oxidative stress in testes of rats subjected to chronic iron intoxication and alpha-tocopherol supplementation. Toxicology 1999; 15: 179–186.
Travacio M, Polo JM, Llesuy S. Chromium (VI) induces oxidative stress in the mouse brain. Toxicology 2000; 162: 139–148.
Usuki F, Yasutake A, Umehara F, Tokunaga H, Matsumoto M, Eto K, Ishiura s, Higuchi I. In vivo protection of a watersoluble derivative of vitamin E, Trolox, against methylmercury-intoxication in the rat. Neurosci. Lett. 2001; 304: 199–203.
Goti D, Hammer A, Galla HJ, Malle E, Sattler W. Uptake of lipoprotein-associated alpha-tocopherol by primary porcine brain capillary endothelial cells. J. Neurochem. 2000; 74: 1374–1783.
Uchida K, Itakura K, Kawakishi S, Hiai H, Toyokuni S, Stadtman ER. Characterization of epitopes recognized by 4-hydroxy-2-nonenal specific antibodies. Arch. Biochem. Biophys. 1995; 324: 241–248.
Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman ER, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc. Natl. Acad. Sci. USA 1996; 93: 2696–2701.
Castellani RJ, Perry G, Siedlak SL, Nunomura A, Shimohama S, Zhang J, Montine T, Sayre LM, Smith MA. Hydroxynonenal adducts indicate a role for lipid peroxidation in neocortical and brainstem Lewy bodies in humans. Neurosci Lett. 2002; 319: 25–28.
Toyokuni S, Uchida K, Okamoto K, Hattori NY, Hiai H, Stadtman ER. Formation of 4-hydroxy-2-nonenal-modified proteins in the renal proximal tubules of rats treated with a renal carcinogen, ferric nitrilotriacetate. Proc. Natl. Acad. Sci. USA 1994; 91: 2616–2620.
Buege JA, Aust SD. Microsomal lipid peroxidation. Methods. Enzymol. 1978; 52: 302–310.
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods. Enzymol. 1990; 186: 464–478.
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [5N] nitrate in biological fluids. Anal. Biochem. 1982; 126: 131–138.
Albery WJ, Boutelle MG, Galley PT. The dialysis electrode—a new method for in vivo monitoring. J. Chem. Soc. Chem. Commun. 1992; 900–901.
Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free. Rad. Biol. Med. 1991; 11: 81–128.
Benedetti A, Comporti M, Esterbauer H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim. Biophys. Acta. 1980; 620: 281–296.
Ando Y, Nyhlin, N, Suhr O, Holmgren G, Uchida K, Sahly MEL, Yamashita T, Terasaki H, Nakamura M, Uchino M, Ando M. Oxidative stress is found in amyloid deposits in systemic amyloidosis. Biochem. Biophys. Res. Commum. 1997; 232: 497–502.
Coombes JS, Powers SK, Demirel HA, Hamilton KL, Jessup J, Vincent HK. Shanely RAVitamin E deficiency fails to affect myocardial performance during in vivo ischemia-reperfusion. Int. J. Vitam. Nutr. Res. 2000; 70: 293–300.
Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, Woodbury P, Growdon J, Cotman CW, Pfeiffer E, Schneider LS, Thal LJ. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N. Engl. J. Med. 1997; 336: 1216–1222.
Grundman M. Vitamin E and Alzheimer disease: the basis for additional clinical trials. Am. J. Clin. Nutr. 2000; 71: 630S-636S.
Patra RC, Swarup D, Dwivedi SK. Antioxidant effects of alpha tocopherol, ascorbic acid and L-methionine on lead induced oxidative stress to the liver, kidney and brain in rats. Toxicology 2001; 162: 81–88.
Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc. Natl. Acad. Sci. USA 1991; 88: 6368–6371.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamashita, T., Ando, Y., Nakamura, M. et al. Inhibitory effect of α-tocopherol on methylmercury-induced oxidative steress. Environ Health Prev Med 9, 111–117 (2004). https://doi.org/10.1007/BF02898069
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02898069