Abstract
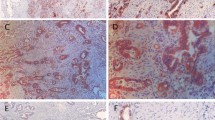
HIF-la induces GLUT-1 expression, and their presence has been evaluated in colorectal cancer. However, the expressions of GLUT-1 and HIF-lα have not been investigated together with reference to clinicopathological characteristics in human colorectal cancer. The aim of our study was to compare the expression of HIF-lα and GLUT-1 with various clinicopathological features of colorectal cancer. The presence of HIF-lα and GLUT-1 was visualized immunohistochemically in 123 primary tumors. Membranous localization of GLUT-1 was found in multifocally necrotizing cancer samples, while pure cytoplasmic perinuclear, mostly supranuclear GLUT-1 accumulation was characteristic of cancer fields with lack of necrosis. HIF-la was located in the cytoplasm and occasionally in the nuclei of cancer cells. Immunoreactivity to GLUT-1 was significantly higher in node-positive cancers compared with nodenegative ones (p=0.04), confirming our earlier results obtained on a larger number of patients. Non-mucinous adenocarcinomas expressed GLUT-1 and HIF-lα with significantly greater frequency than mucinous adenocarcinomas (p=0.002, p=0.0002, respectively). GLUT-1 and HIF-la expression did not differ in relation to tumor stage, location, or patients′ age or gender. In contrast to that of GLUT-1, expression of HIF-lα correlated with grade (p=0.00003) without difference with regard to pN status. HIF-lα expression correlated with GLUT-1 expression in the whole patient population, as well as in all clinicopathological groups except for the pTl+pT2 group. Although the coexpression of cytoplasmic HIF-la and GLUT-1 does not directly prove the dependence between HIF-1 as a nuclear transcriptional factor and GLUT-1 as its downstream protein, it is evidence of their simultaneous upregulation. The extranuclear accumulation of HIF-la and GLUT-1 requires further studies to explain its significance in colorectal cancer.
Similar content being viewed by others
References
Wang GL, Jiang BH, Rue EA, Semenza GL: Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by ceUular O2 tension. Proc Natl Acad Sci USA 92: 5510–5514, 1995
Wood SM, Wiesener MS, Yeates KM, Okada N, Pugh CW, Maxwell PH, Ratcliffe PJ: Selection and analysis of a mutant cell line defective in the hypoxia-inducible factor-1 alpha-subunit (HIF-lalpha). Characterization of hif-1 alpha-dependent and -independent hypoxia-inducible gene expression. J Biol Chem 273: 8360–8368, 1998
Allen JW, Johnson RS, Bhatia SN: Hypoxic inhibition of — methylcholanthrene-induced CYP1A1 expression is independent of HIF-lalpha. Toxicol Lett 155: 151–159, 2005
Chen J, Zhao S, Nakada K, Kuge Y, Tamaki N, Okada F, Wang J, Shindo M, Higashino F, Takeda K, Asaka M, Katoh H, Sugiyama T, Hosokawa M, Kobayashi M: Dominant-negative hypoxia-inducible factor-1 alpha reduces tumorigenicity of pancreatic cancer cells through the suppression of glucose metabolism. Am J Pathol 162: 1283–1291, 2003
Williams KJ, Telfer BA, Airley RE, Peters HP, Sheridan MR, van der Kogel AJ, Harris Ah, Stratford IJ: A protective role for HIF-1 in response to redox manipulation and glucose deprivation: implications for tumorigenesis. Oncogene 21: 282–290, 2002
Vleugel MM, Greijer AE, Shvarts A, van der Groep P, van Berkel M, Aarbodem Y, van Tinteren H, Harris AL, van Diest PJ, van der Wall E: Differential prognostic impact of hypoxia induced and diffuse HIF-lalpha expression in invasive breast cancer. J Clin Pathol 58: 172–177, 2005
Kunkel M, Reichert TE, Benz P, Lehr HA, Jeong JH, Wieand S, Bartenstein P, Wagner W, Whiteside TL: Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer 97: 1015–1024, 2003
Yoshimura H, Dhar DK, Kohno H, Kubota H, Fujii T, Ueda S, Kinugasa S, Tachibana M, Nagasue N: Prognostic impact of hypoxia-inducible factors 1alpha and 2alpha in colorectal cancer patients: correlation with tumor angiogenesis and cyclooxygenase-2 expression. Clin Cancer Res. 10: 8554–8560, 2004
Cooper R, Sarioglu S, Sokmen S, Fuzun M, Kupelioglu A, Valentine H, Gorken IB, Airley R, West C: Glucose transporter1 (GLUT-1): a potential marker of prognosis in rectal carcinoma? Br J Cancer 89: 870–876, 2003
Furudoi A, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F: Clinical significance of human erythrocyte glucose transporter 1 expression at the deepest invasive site of advanced colorectal carcinoma. Oncology 60: 162–169, 2001
Haber RS, Rathan A, Weiser KR, Pritsker A, Itzkowitz SH, Bodian C, Slater G, Weiss A, Burstein DE: GLUT1 glucose transporter expression in colorectal carcinoma: a marker for poor prognosis. Cancer 83: 34–40, 1998
Palit V, Phillips RM, Puri R, Shah T, Bibby MC: Expression of HIF-lalpha and Glut-1 in human bladder cancer. Oncol Rep 14: 909–913, 2005
Koukourakis MI, Giatromanolaki A, Harris AL, Sivridis E: Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: a metabolic survival role for tumor-associated stroma. Cancer Res 66: 632–637, 2006
Wincewicz A, Sulkowska M, Koda M, Kanczuga-Koda L, Witkowska E, Sulkowski S: Significant co-expression of GLUT-1, Bcl-xl and Bax in colorectal cancer. Ann N Y Acad Sci 2007 (accepted)
Fogt F, Wellmann A, Urbanski SJ, Noffsinger A, Poremba C, Zimmerman RL, Alsaigh N: Glut-1 expression in dysplastic and regenerative lesions of the colon. Int J Mol Med 7: 615–619, 2001
Ito H, Duxbury M, Zinner MJ, Ashley SW, Whang EE: Glucose transporter-1 gene expression is associated with pancreatic cancer invasiveness and MMP-2 activity. Surgery 136: 548–556, 2004
Sakashita M, Aoyama N, Minami R, Maekawa S, Kuroda K, Shirasaka D, Ichihara T, Kuroda Y, Maeda S, Kasuga M: Glutl expression in Tl and T2 stage colorectal carcinomas: its relationship to clinicopathological features. Eur J Cancer 37: 204–209, 2001
Younes M, Juarez D, Lechago LV, Lerner SP: Glut 1 expression in transitional cell carcinoma of the urinary bladder is associated with poor patient survival. Anticancer Res 21: 575–578, 2001
Grover-McKay M, Walsh SA, Seftor EA, Thomas PA, Hendrix MJ: Role for glucose transporter 1 protein in human breast cancer. Pathol Oncol Res 4: 115–120, 1998
Airley R, Loncaster J, Davidson S, Bromley M, Roberts S, Patterson A, Hunter R, Stratford I, West C: Glucose transporter glut-1 expression correlates with tumor hypoxia and predicts metastasis-free survival in advanced carcinoma of the cervix. Clin Cancer Res 7: 928–934, 2001
Bos R, van der Groep P, Greijer AE, Shvarts A, Meijer S, Pinedo HM, Semenza GL, van Diest PJ, van der Wall E: Levels of hypoxia-inducible factor-1alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer 97: 1573–1581, 2003
Couvelard A, O’Toole D, Turley H, Leek R, Sauvanet A, Degott C, Ruszniewski P, Belghiti J, Harris AL, Gatter K, Pezzella F: Microvascular density and hypoxia-inducible factor pathway in pancreatic endocrine tumours: negative correlation of microvascular density and VEGF expression with tumour progression. Br J Cancer 92: 94–101, 2005
Garofalo C, Koda M, Cascio S, Sulkowska M, Kanczuga-Koda L, Golaszewska J, Russo A, Sulkowski S, Surmacz E: Increased expression of leptin and the leptin receptor as a marker of breast cancer progression: possible role of obesity-related stimuli. Clin Cancer Res 12: 1447–1453, 2006
Sivridis E, Giatromanolaki A, Gatter KC, Harris AL, Koukourakis MI;Tumor and Angiogenesis Research Group: Association of hypoxia-inducible factors 1alpha and 2alpha with activated angiogenic pathways and prognosis in patients with endometrial carcinoma. Cancer 95: 1055–1063, 2002
Yoo YG, Yeo MG, Kim DK, Park H, Lee MO: Novel function of orphan nuclear receptor Nur77 in stabilizing hypoxia-inducible factor-1alpha. J Biol Chem 279: 53365–53373, 2004
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wincewicz, A., Sulkowska, M., Koda, M. et al. Clinicopathological significance and linkage of the distribution of HIF-1α and GLUT-1 in human primary colorectal cancer. Pathol. Oncol. Res. 13, 15–20 (2007). https://doi.org/10.1007/BF02893436
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02893436