Abstract
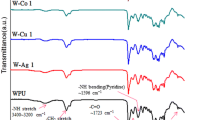
A series of waterborne poly(urethane-urea) anionomers were prepared from isophorone diisocyanate (IPDI), polycaprolactone diol (PCL), dimethylol propionic acid (DMPA), ethylene diamine (EDA), and triethylamine (TEA), NaOH, or Cu(COOCH3)2 as neutralizing agent. This study was performed to decide the effect of neutralizing agent type on the particle size, viscosity, hydrogen bonding index, adhesive strength, antistaticity, antibacterial and mechanical properties. The particle size of the dispersions decreased in the following order: TEA based samples (T-sample), NaOH based samples(N-sample), and Cu(COOCH3)2 based sample (C-sample). The viscosity of the dispersions increased in the order of C-sample, N-sample, and T-sample. Metal salt based film samples (N and C-sample) had much higher antistaticity than TEA based sample. By infrared spectroscopy, it was found that the hydrogen bonding index (or fraction) of samples decreased in the order of T-sample, N-sample, and C-sample. The adhesive strength and tensile modulus/strength decreased in the order of T-sample, N-sample, and C-sample. The C-sample had strong antibacterial halo, however, T- and N-samples did not.
Similar content being viewed by others
References
J. A. Miller, K. K. S. Hwang, and S. L. Cooper,J. Macromol. Sci. Phys.,B22(2), 321 (1983).
J. T. Koberstein and R. S. Stein,J. Polym. Sci., Polym. Phys.,21, 1439 (1983).
A. Rembaum, H. Rile, and R. Somoano,J. Polym. Sci.,138, 457 (1970).
A. Rembaum, S. D. S. Yen, R. F. Randal, and M. Shen,J. Macromol. Sci.,A4(3), 715 (1970).
H. A. Salah, K. C. Frisch, H. X. Xiao, and J. A. Mclean,J. Polym. Sci., Polym. Chem.,25, 2127 (1987).
O. Lorenz and H. Hick,Angew. Macromol. Chem.,72, 115 (1978).
I. Dimitrievski, T. Malavasic, U. Osdredkar, and I. Vizovisek,Vestn. Slov. Kem. Drus.,35(3), 253 (1988).
K. K. S. Hwang, C. Z. Yang, and S. L. Cooper,Polym. Eng. Sci.,21, 1027 (1981).
C. Geraldine and A. Eisenberg,Ind. Eng. Chem. Prod. Res. Dev.,20, 271 (1981).
Y. Chen and Y. L. Chen,J. Appl. Polym. Sci.,46(3), 435 (1992).
O. Lorenz, H. J. August, H. Hick, and F. Triekes,Angew. Makromol. Chem.,63, 11 (1977).
D. Dieterich, G. Belle, and H. Schmeiner,Ger. Offen.,2, 735 (1979).
D. J. Hourston, G. D. Williams, R. Satguru, J. C. Padget, and D. Pears,J. Appl. Polym. Sci.,74(3), 556 (1999).
M. M. Coleman, D. J. Skrovanek, J. Hu, and P. C. Painter,Macromolecules,21, 59 (1988).
T. C. Wen and M. S. Wu,Macromolecules,32, 2712 (1999).
C. S. P. Sung and N. S. Schneider,Macromolecules,10, 452 (1977).
S. K. Pollack, D. Y. Shen, S. L. Hsu, Q. Wang, and H. D. Stidham,Macromolecules,22, 551 (1989).
R. W. Coleman, G. M. Ester, and S. L. Cooper,Macromolecules,3(5), 579 (1970).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, JE., Lee, YH., Koo, YS. et al. Preparation and properties of waterborne poly(urethane-urea) ionomers effect of the type of neutralizing agent. Fibers Polym 3, 97–102 (2002). https://doi.org/10.1007/BF02892624
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02892624