Summary
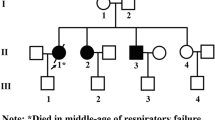
A newborn female, the second child of consanguineous parents, exhibited general muscle hypotonia, apathy, hepatomegaly and failure to thrive from birth and signs of craniofacial dysmorphia were present. Pipecolic and trihydroxicoprostanoic acid were excreted in the urine and serum transferrin, ferritin and iron were markedly elevated. At the age of 7 weeks the baby died of respiratory insufficiency.
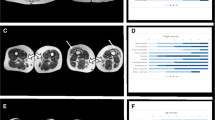
Besides malformations of the brain, renal cysts, liver damage with hypoplastic intrahepatic bile ducts and cholestasis, increased storage of iron and cytochemically proven deficiency of peroxisomes in liver and kidney, morphological studies provided evidence of a mitochondrial myopathy in striated muscle with the accumulation of enlarged bizarre mitochondria, showing only minor structural abnormalities. No defects of NADH-reductase, succinate-dehydrogenase or cytochrome-c-oxidase were demonstrated histochemically. Cytochemical-ultrastructural investigation of mitochondrial ATPase revealed activation of the ATP-synthesising enzyme even before the addition of an uncoupler, this indicating loosely coupled oxidative phosphorylation. In addition a high rate of subcellular autophagy with segregation of mitochondria and focal loss of fibrils was present.
Muscle damage in Zellweger syndrome appears to be the consequence of complex, interacting metabolic processes. The mitochondrial myopathy thereby induced allows a better understanding of general muscle hypotonia, one of the leading symptoms of this disorder.
Similar content being viewed by others
References
Arneson DW, Tipton RE, Ward JC (1982) Hyperpipecolic acidemia. Occurence in an infant with clinical findings of the cerebrohepatorenal (Zellweger) syndrome. Arch Neurol 39:713–716
Böck P, Kramar R, Pavelka M (1980) Peroxisomes and related particles in animal tissue. Cell Biol Monographs, vol 7. Springer, Wien New York
Borst P, Loos JA, Christ EJ, Slater EC (1962) Uncoupling activity of long-chain fatty acids. Biochim Biophys Acta 62:509–518
Borchard F, Becker K, Müntefering H, Braun D, Wechsler W, Bremer HS (1982) Morphological findings in 3 children with Zellweger’s syndrome with special regard to cytochemical investigations. Verh Dtsch Ges Pathol 66:422–429
Brown III FR, McAdams AJ, Cummins JW, Konkol R, Singh I, Moser AB, Moser HW (1982) Cerebro-hepato-renal (Zellweger) syndrome and neonatal adrenoleukodystrophy: similarities in phenotype and accumulation of very long chain fatty acids. Johns Hopk Med J 151:344–361
Brun A, Gilboa M, Meeuwisse GW, Nordgren H (1978) The Zellweger syndrome: Subcellular pathology, neuropathology, and the demonstration of pneumocystis carinii pneumonitis in two siblings. Eur J Pediatr 127:229–245
Carlson BR, Weinberg AG (1978) Giant cell transformation. Cerebro-hepatorenal syndrome. Arch Pathol Lab Med 102:596–599
Chan SHP, Higgins E (1978) Uncoupling activity of endogenous free fatty acids in rat liver mitochondria. Can J Biochem 56:111–116
DiMauro S, Mendell JR, Sahenk Z, Bachman D, Scarpa A, Scofield RM, Rainer C (1980) Fatal infantile mitochondrial myopathy and renal dysfunction due to cytochrome-c-oxidase deficiency. Neurology 30:795–804
Endres W, Müller-Höcker J, vd Ende A, Schutgens R BH, Bise K, Hübner G, Wadman SK (1981) Cerebro-hepato-renal syndrome of Zellweger. Absence of liver peroxisomes, hypocatalasia and renal excretion of pipecolic and trihydroxycoprostanoic acid. Eur J Pediatr [Abstract] 135:331
Ernster L, Schatz G (1981) Mitochondria: a historical review. J Cell Biol 91:227–255
Garzuly F, Szabo L, Kadas L (1974) Neuronale Migrationsstörung bei cerebrohepato-renalem Syndrom “Zellweger”. Neuropädiatrie 5:318–328
Gilchrist KW, Gilbert EF, Goldfarb S, Goll U, Spranger JW, Opitz JM (1976) Studies of malformation syndromes of man XI B: The cerebro-hepatorenal syndrome of Zellweger: Comparative pathology. Eur J Pediatr 121:99–118
Goldfischer S (1979) Peroxisomes in Disease. J Histochem Cytochem 27:1371–1373
Goldfischer S (1982) Peroxisomes and human metabolic diseases: The cerebrohepatorenal syndrome (CHRS), cerebrotendinous xanthomatosis, and Schilder’s disease (adrenoleuko-dystrophy). Ann NY Acad Sci 386:526–529
Goldfischer S, Moore CL, Johnson AB, Spiro AJ, Valsamis MP, Wisniewski HK, Ritch RH, Norton WT, Rapin J, Gartner LM (1973) Peroxisomal and mitochondrial defects in the cerebro-hepato-renal syndrome. Science 182:62–64
Goldfischer S, Powers JM, Johnson AB, Axe S, Brown FR, Moser HW (1983) Striated adrenocortical cells in cerebro-hepato-renal (Zellweger) syndrome. Virchows Arch [Pathol Anat] 401:355–361
Govaerts L, Monnens L, Tegelaers W, Trijbels F, v Raay-Selten A (1982) Cerebro-Hepato-renal syndrome of Zellweger: clinical symptoms and relevant laboratory findings in 16 patients. Eur J Pediatr 139:125–128
Hajra AK, Bishop JE (1982) Glycerolipid biosynthesis in peroxisomes via the acyl dihydroxiaceton phosphate pathway. In: Kindl H, Lazarow PB (eds) Peroxisomes and glyoxisomes. Ann New York Acad Science 386:170–182
Hanson RF, Szczepanik-Vanleeuwen P, Williams GC, Grabowski G, Sharp HL (1979) Defects of bile acid synthesis in Zellweger’s syndrome. Science 203:1107–1108
Heiman-Patterson TD, Bonilla E, DiMauro S, Foreman J, Schottland DL (1982) Cytochrome-c-oxidase deficiency in a floppy infant. Neurology 32:898–900
ba]Hruban Z, Rechcigl M (1969) Microbodies and related particles. Int Rev Cytol [Suppl I]
Hruban Z, Vigil EL, Slesers A, Hopkins E (1972) Microbodies: Constituent organelles of animal cells. Lab Invest 27:184–191
Kindl H, Lazarow PB (1982) Peroxisomes and Glyoxisomes. Ann New York Acad Science 386
Kohn R, Mundel G (1969) Cerebro-hepato-renal syndorme. Report of a case. Helv Paediat Acta 24:352–360
Lee MJ, Whitehouse MW (1965) Inhibition of electron transport and coupled phosphorylation in liver mitochondria by cholanic (bile) acids and their conjugates. Biochem Biophys acta loo: 317–328
Liu HM, Bangaru BS, Kidd J, Boggs J (1976) Neuropathological considerations on cerebrohepato-renal syndrome (Zellweger’s syndrome). Acta Neuropathol 34:115–123
Lott IT, Mathis RK, Carter EA, Watkins JB (1979) Mitochondrial abnormalities in the cerebrohepato-renal syndrome. Ann Neurol [Abstract] 6:173–174
Masters CJ, Holmes RS (1977a) The metabolic roles of peroxisomes in mammalian tissues. Int J Biochem 8:549–553
Masters CJ, Holmes RS (1977b) Peroxisomes: New aspects of cell physiology and biochemistry. Physiol Rev 57:816–882
Mathis RK, Watkins JB, Szczepanik-v. Leeuween P, Lott IT (1980) Liver in the cerebro-hepatorenal syndrome: defective bile acid synthesis and abnormal mitochondria. Gastroenterology 79:1311–1317
Meijer AEFH (1977) Skeletal muscle diseases with disordered oxidative phosphorylation in the isolated muscle mitochondria. Cell Mol Biol 23:257–265
Meijer AEFH (1983) The histochemical demonstration of loosely coupled mitochondria in human skeletal muscle. Histochem J 15:331–335
Meijer AEFH, Vloedman AHT (1980) The histochemical characterization of the coupling state of skeletal muscle mitochondria. Histochemistry 69:217–232
Mellors A, Tappel AL, Sawant PL, Desai ID (1967) Mitochondrial swelling and uncoupling of oxidative phosphorylation by lysosomes. Biochem Biophys Acta 143:299–309
Mitchell P (1966) Chemoosmotic coupling in oxidative and photosynthetic phosphorylation. Br Rev Camb Phil Soc 41:445–502
Mitchell P (1977) Vectorial chemoosmotic processes. Ann Rev Biochem 46:996–1005
Monnens L, Bakkeren J, Parmentier G, Janssen G, van Haelst U, Trijbels F, Eyssen H (1980) Disturbances in bile acid metabolism of infants with the Zellweger (cerebro-hepato-renal) syndrome. Eur J Pediatr 133:31–35
Monnens L, Bakkeren J, Parmentier G, Janssen G, van Haelst U, Trijbels F, Eyssen H (1980) Disturbances in bile acid metabolism of infants with the Zellweger (cerebro-hepato-renal) syndrome. Eur J Pediatr 133:31–35
Müller-Höcker J, Bise K, Endres W, Hübner G (1981) Morphology and Diagnosis of Zellweger Syndrome. A contribution to combined cytochemical-finestructural identification of peroxisomes in autopsy material and frozen liver tissue with case report. Virchows Arch [Pathol Anat] 393:103–114
Müller-Höcker J, Pongratz D, Deufel Th, Trijbels JMF, Endres W, Hübner G (1983) Fatal lipid storage myopathy with deficiency of cytochrome-c-oxidase and carnitine. A contribution to the combined cytochemical-finestructural identification of cytochrome-c-oxidase in longterm frozen muscle. Virchows Arch [Pathol Anat] 399:11–23
Passarge E, McAdams AJ (1967) Cerebro-hepato-renal syndrome. A newly recognized hereditary disorder of multiple congenital defects including sudanophilic leukodystrophy, cirrhosis of the liver, and polycystic kidneys. J Pediatr 71:691–702
Petersen JI, Gustafsson J (1980) Conversion of 3alpha, 7alpha, 12alpha-trihydroxi-5beta-cholestanoic acid into cholic acid by rat liver peroxisomes. FEBS Lett 121:345–348
Pfeifer U, Sandhage K (1979) Licht- und elektronenmikroskopische Leberbefunde beim cerebro-hepato-renalen Syndrom nach Zellweger (Peroxisomendefizienz). Virchows Arch [Pathol Anat] 384:269–284
Smith DW, Opitz JM, Inhorn SL (1965) A syndrome of multiple developmental defects including polycystic kidneys and intrahepatic biliary dysgenesis in 2 siblings. J Pediatr 67:617–624
Stansbie D, Dormer RL, Hughes IA, Minchom PE, Hendry GAF, Jones OTG, Cross AR, Sherratt HSA, Turnbull DM, Johnson MA (1982) Mitochondrial myopathy with skeletal muscle cytochrome-c-oxidase deficiency. J Inherited Metab Dis [Suppl 5] 1:27–28
Sternlieb I, Quintana N (1977) The Peroxisomes of human hepatocytes. Lab Invest 36:140–149
Tolbert NE, Essner E (1981) Microbodies and glyoxisomes. J Cell Biol 91:271s-283s
Trijbels JMF, Monnens LAH, Bakkeren JAJM, Van Raay-Selten AHJ, Corstiaensen JMB (1979) Biochemical studies in the cerebro-hepato-renal syndrome of Zellweger: A disturbance in the metabolism of pipecolic acid. J Inherited Metab Dis 2:39–42
Trijbels JMF, Berden JA, Monnens LAH, Willems JL, Janssen AJM, Schutgens RBH, van dBroek-van Essen M (1983) Biochemical studies in the liver and muscle of patients with Zellweger syndrome. Pediatr Res 17:514–517
Van Biervliet JP, Bruinvis L, Ketting D, De Bree PK, vd Heiden C, Wadman SK, Willems JL, Bookelman H, V Haelst U, Monnens LA (1977) Hereditary mitochondrial myopathy with lactic acidemia, a De Toni-Fanconi-Debre syndrome, and a defective respiratory chain in voluntary striated muscles. Pédiatric Res 11:1088–1092
Versmold HT, Bremer HJ, Herzog V, Siegel G, v. Bassewitz DB, Irle U, v. Voss H, Lombeck J, Brauser B (1977) A metabolic disorder similar to Zellweger syndrome with hepatic acatalasia and absence of peroxisomes, altered content and redox state of cytochromes, and infantile cirrhosis with hemosiderosis. Eur J Peidatr 124:261–275
Volpe JJ, Adams RD (1972) Cerebro-hepato-renal syndrome of Zellweger: An inherited disorder of neuronal migration. Acta Neuropathol 2:175–198
Zborowski J, Wojtczak KL (1963) Induction of swelling of liver mitochondria by fatty acids of various chain length. Biochem Biophys Acta 70:596
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Müller-Höcker, J., Walther, J.U., Bise, K. et al. Mitochondrial myopathy with loosely coupled oxidative phosphorylation in a case of Zellweger Syndrome. Virchows Archiv B Cell Pathol 45, 125–138 (1984). https://doi.org/10.1007/BF02889859
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02889859