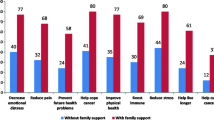
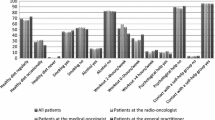
Abstract
Background: Despite the popularity and widespread practice of complementary/alternative medicine (CAM), researchers may face problems accruing patients to randomized clinical trials, considered the gold standard of biomedical research. Strict exclusion criteria and barriers to participation may limit accrual. Inadequate numbers of subjects decrease the ability of studies to detect an effect that exists and generalize their findings. This article describes the recruitment experience of a CAM trial, details reasons for non-participation and contrasts participants and non-participants on demographic, clinical, and treatment-related variables. Methods: Women who were Houston area residents and spoke English, had primary breast cancer (excluding Stage IV), and were 1 to 30 months posttreatment with no steroids, tamoxifen, substance abuse, psychiatric or heart disease, or immune deficiency were eligible. The enrollment process involved three contacts (i.e. introductory letter and brochure, telephone calls, and reminder post cards). Potential participants were told that the study would require blood samples (30cc) to assess immune function; psychosocial measures to assess emotional well-being, quality-of-life, social support, and coping strategies; and possible assignment to six weekly support or imagery sessions. Factors influencing recruitment and reasons for non-participation were assessed by stratified analysis and multivariate logistic regression. Results: Of 158 eligible participants, 30% (N=47) consented to participate. Primary reasons for non-participation included work/childcare (33.3%), transportation/travel (30.6%), and lack of interest (24.3%). Participants were more likely to be 40–54 years of age versus younger or older, divorced/separated, and able to pay some/all medical expenses. Divorced or separated women appeared to be more likely to participate, regardless of financial status. Conclusion: Researchers must assess the impact of exclusion criteria on accrual and recognize the special needs of their target population. Although age, marital status, and pay status were the strongest predictors of participation, these factors are not amenable to intervention. Based on this study, researchers might boost accrual by providing interventions available during the day and evening to accommodate working women, child care services, transportation, or reimbursement for travel costs.
Similar content being viewed by others
References
Eisenberg D, Kessler RC, Foster C, et al: Unconventional medicine in the United States: Prevalence, costs, and patterns of use.New England Journal of Medicine. 1993,328: 246–252.
Cassileth BR, Lusk EF, Strouse TB, Bodenheimer BJ: Contemporary unorthodox treatments in cancer medicine: A study of patients, treatments, and practitioners.Annals of Internal Medicine. 1984,101: 105–112.
American Cancer Society: Unproven methods of cancer management-Macrobiotic diets for the treatment of cancer.CA: A Cancer Journal for Clinicians. 1989,39(4): 248–251.
Lerner IJ, Kennedy BJ: The prevalence of questionable methods of cancer treatments in the United States.Cancer. 1992,42: 181–191.
U.S. House Select Committee on Aging: Quackery: A Ten Billion Dollar Scandal. 98th Congress, 2nd Session: May 31, 1984. Washington, DC: U.S. Government Printing Office.
Herbert V: Unproven (questionable) dietary and nutritional methods in cancer prevention and treatment.Cancer. 1986,58: 1930–1941.
Ernst E: Complementary cancer treatments: Hope or hazard?Clinical Oncology. 1995,7: 259–263.
Gordon J: Alternative medicine and the family physician.American Family Physician. 1996,54(7): 2205–2212.
Richardson MA: Alternative cancer therapies: Facilitating information and evaluations. Third Annual International Congress on Alternative and Complementary Therapies. Washington, DC: September, 1997.
McGinnis LS: Alternative therapies, 1990. An overview.Cancer. 1991,67: 1788–1793.
Cassileth BR: Unorthodox cancer medicine.Cancer Investigation. 1986,4: 591–598.
Mock V, Hill MN, Dienemann JA, Grimm PM, Shivnan JC: Challenges to behavioral research in oncology.Cancer Practice. 1996,4(5): 267–273.
Hunningshake DB, Darby CA, Probstfield JL: Recruitment experience in clinical trials: Literature summary and annotated bibliography.Controlled Clinical Trials. 1987,9(Suppl.): 6S-30S.
Leventhal H, Nerenz DR, Leventhal EA, Love RR, Bendena LM: The behavioral dynamics of clinical trials.Preventive Medicine. 1991,20: 132–146.
Daly D: Alternative medicine courses taught at U.S. medical schools: An ongoing listing.Journal of Alternative and Complementary Medicine. 1996,2: 315–317.
Weil A: The body’s healing systems: The future of medical education.Alternative and Complementary Therapies. 1995,September: 305–310.
Ernst E, Resch K, White AR: Complementary medicine: What physicians think of it—A meta-analysis.Archives of Internal Medicine. 1995,155: 2405–2408.
Goodwin J: A health insurance revolution.New Age Journal. 1997,April: 95–99.
Weber DO: The mainstreaming of alternative medicine.Healthcare Forum Journal. 1966,Nov/Dev: 16–27.
Office of Alternative Medicine Clearing House-NIH: OAM highlights strategic plan for AMPAC.Complementary and Alternative Medicine at the NIH. 1997,4(4): 1.
Winn RJ: Obstacles to the accrual of patients to clinical trials in the community setting.Seminars in Oncology. 1994,21(4): 112–117.
Gotay CC: Accrual to cancer clinical trials: Directions from the research literature.Social Science and Medicine. 1991,33(5): 569–577.
Winn RJ, Miransky J, Kerner JF, et al (eds): An evaluation of physician determinants in the referral of patients for cancer clinical trials in the community setting. InAdvances in Cancer Control: Epidemiology and Research. New York: Liss, 1984, 63–73.
Benson AB, Pregler JP, Bean JA, et al: Oncologists’ reluctance to accrue patients onto clinical trials: An Illinois cancer center study.Journal of Clinical Oncology. 1991,9: 2607.
Mackillop WJ, Ward GK, O’Sullivan B: The use of expert surrogates to evaluate clinical trials in non-small cell lung cancer.British Journal of Cancer. 1986,54: 661–667.
Moore JJ, O’Sullivan B, Tannack IF: How expert physicians would wish to be treated if they had genitourinary cancer.Journal of Clinical Oncology. 1988,6: 1736–1745.
Taylor KM, Klener M: Informed consent: A physicians’ perspective.Social Science and Medicine. 1987,24: 135–147.
Penman DT, Holland JC, Bahna GF, et al: Informed consent for investigational chemotherapy: Patients’ and physicians’ perceptions.Journal of Clinical Oncology. 1984,2(7): 849–855.
Boring CC, Quires TS, Tong T: Cancer statistic.CA: A Cancer Journal for Clinicians. 1991,41: 19–36.
Barofsky I, Sugarbaker PH: Determinants of patient nonparticipation in randomized clinical trial for the treatment of sarcomas.Cancer Clinical Trials. 1979,2: 237–246.
Hunter CP, Frelick RW, Feldman AR: Selection factors in clinical trials: Results from the community clinical oncology program physician’s patient log.Cancer Treatment Reports. 1987,71: 559–565.
Kemp N, Skinner E, Toms J: Randomized clinical trials of cancer treatment—A public opinion survey.Clinical Oncology. 1984,10: 155–161.
Llewellyn-Thomas HA, McGreal MJTE, Fine S, Erlichman C: Patients willingness to enter clinical trials: Measuring the association with perceived benefit and preference for decision participation.Social Science and Medicine. 1991,32: 35–42.
Sutherland HJ, Llewellyn-Thomas HA, Lockwood GA, Trichler DL, Till JE: Cancer patients: Their desire for information and participation in treatment decisions.Journal of Royal Society of Medicine. 1989,82: 260–263.
Mettlin C, Cummings KM, Walsh D: Risk factor and behavioral correlates of willingness to participate in cancer prevention trials.Nutrition and Cancer. 1985,7: 189–198.
Yeomans-Kinney A, Vernon SW, Frankowski RF, et al: Factors related to enrollment in the breast cancer prevention trial at a comprehensive cancer center during the first year of recruitment.Cancer. 1995,76(1): 45–56.
Daly M, Seay J, Balshem A, Lerman C, Engstrom P: Feasibility of a telephone survey to recruit health maintenance organization members into a tamoxifen chemoprevention trial.Cancer Epidemiology, Biomarkers and Prevention. 1992,1: 413–416.
Hudman KS, Yeomans AY: Issues of enrollment bias in cancer chemoprevention trials.Cancer Bulletin. 1995,47: 339–342.
Lerman C, Rimer BK, Daly M, et al: Recruiting high risk women into a breast cancer health promotion trial.Cancer Epidemiology, Biomarkers and Prevention. 1994,3: 271–276.
Deans G, Bennett-Emslie GB, Weir J, Smith DC, Kaye SB: Cancer support groups—Who joins and why?British Journal of Cancer. 1988,58: 670–674.
Bauman LJ, Gervey R, Siegel K: Factors associated with cancer patients’ participation in support groups.Journal of Psychosocial Oncology. 1992,10(3): 1–20.
Bresberg BA, Levenson JL: A survey of cancer support groups provided by the National Cancer Institute (NCI) clinical and comprehensive centers.Psycho-Oncology. 1993,2: 215–217.
Loescher LJ, Graham VE, Aickin M, Mcyskens FL, Surwit EA: Development of a contingency recruitment plan for a phase III chemoprevention trial of cervical dysplasia.Progress in Clinical and Biological Research. 1990,339: 1551–1563.
Fisher B: On clinical trial participation.Journal of Clinical Oncology. 1991,9: 1927–1930.
Begg CB, Engstromm PF: Eligibility and extrapolation in cancer clinical trials.Journal of Clinical Oncology. 1987,5: 962–968.
Spiegel D, Bloom JR: Group therapy and hypnosis reduces metastatic breast carcinoma pain.Psychosomatic Medicine. 1983,45: 333–339.
Fawzy FI, Fawzy NW, Hyun CS, et al: Effects of an early structured psychiatric intervention.Archives of General Psychiatry. 1993,50: 681–689.
Douglas S, Briones J, Chronister C: The incidence of reporting consent rates in nursing research articles.Image: The Journal of Nursing Scholarship. 1993,26(1): 35–40.
Cassileth BR, Lusk EJ, Miller DS, Hurwitz S: Attitudes toward clinical trials among patients and the public.Journal of the American Medical Association. 1982,246(8): 968–970.
Pocock SJ: Size of cancer clinical trials and stopping rules.British Journal of Cancer. 1978,38: 757–766.
Jack WJL, Chetty U, Rodger A: Recruitment to a prospective breast conservation trial: Why are so few patients randomized?British Medical Journal. 1991,301: 82–85.
Moye LA, Richardson MA, Post-White J, Justice B: Research methodology in psychoneuroimmunology: Rationale and design of the IMAGES-P Clinical Trial.Alternative Therapies in Health and Medicine. 1995,1(2): 34–39.
Hermanek P, Sobin LH (eds):TNM Classification of Malignant Tumors. Berlin, Germany: Springer Verlag, 1987.
Vinokur AD, Threatt BA, Caplan RD, Zimmerman BL: Physical and psychosocial functioning and adjustment to breast cancer: Longterm follow-up of a screening population.Cancer. 1989,63: 394–405.
Bal DG: Cancer in African-Americans.CA: A Cancer Journal for Clinicians. 1992,42(1): 5–17.
Miller HH, Bauman LJ, Friedman DR, DeCosse JJ: Psychosocial adjustment of familial polyposis patients and participation in a chemoprevention trial.International Journal of Psychiatry in Medicine. 1986,16(3): 211–230.
Levy SM, Haynes LT, Herberman RB, et al: Mastectomy versus breast conservation surgery: Mental health effects at long-term follow-up.Health Psychology. 1992,11(6): 349–354.
Morrow GR, Hickok JT, Burish TG: Behavioral aspects of clinical trials: An integrated framework from behavioral theory.Cancer. 1994,74(Suppl.): 2676–2682.
Borhani NO, Tonascia J, Schlundt DG, Prineas RJ, Jefferys JL: Recruitment in the hypertension prevention trial.Controlled Clinical Trials. 1989,10(Suppl.): 30–39.
Author information
Authors and Affiliations
Additional information
Preparation of this manuscript was supported in part by an NIH Office of Complementary/Alternative Medicine Grant #1-R21-RR09505-01 and an NCI Predoctoral Fellowship Grant #R25-57730.
The authors thank Mary Tripp, M.P.H., Alice Madary, R.N., and Elaine White, M.P.H. at The University of Texas M.D. Anderson Cancer Center for assistance with recruitment, screening, and protocol development.
About this article
Cite this article
Richardson, M.A., Post-White, J., Singletary, S.E. et al. Recruitment for complementary/alternative medicine trials: WHO participates after breast cancer. ann. behav. med. 20, 190–198 (1998). https://doi.org/10.1007/BF02884960
Issue Date:
DOI: https://doi.org/10.1007/BF02884960