Abstract
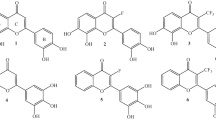
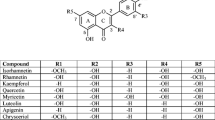
AM1 method was emyloyed to calculate flavonoid antioxidants, and the results obtained are as follows. Firstly, flavonoid hydroxyls atortho position were more active than the hydroxyls atmeta position in scavenging oxygen-free raidicals, which resulted from the facts that (i) the former were stabilized by forming intramolecular hydrogen bond and (ii)ortho benzocluinone formed in the former structures through resonance, which resulted in large percentage of distribution of spin density or1ortho oxygen and low internal energy. Secondly, electron-attracting effect of ring C of chromone-flavonoids showed some passive efftrts on hydroxyls of ring A, making the OH less active. As ring C had little effect on ring B and hydroxyls of ring B in most flavonoids were atortho position, the rule summarized from experiments showing that hydroxyls of ring B were more active in scavenging oxygen-free radicals was elucidated.
Similar content being viewed by others
References
Liu, W. L. (ed.),Concise Biophysics (in Chinese), Beijing:Higher Education Press, 1995, 138.
Cao, G. F., Werlg, X. C., Studies on oxidation stability of fish oil (I),China Oils and Fats (in Chinese), 1995, 20(4): 49.
Cao, G. F., Werng, X. C., Studies on oxidation stability of fish oil (II),China Oils and Fats (in Chinese), 1995, 20(5): 44.
Xin, W. J., Zhao, B. L., Li, X. J. et al., Scavenging effects of Chinese herbs and natural health products on active oxygen radicals,Res. Chem. Intermed., 1990, 14: 171.
Harkme, J. B. (ed.),The Flavonoids:Advances in Research Since 1980, London:Chapman & Hall Press, 1988.
Harborne, J. B. (ed.),The Flavonoids:Advances in Research Since 1986, London:Chapman & Hall Press, 1994.
Wang, X. K. (ed.),Medicinal Chemistry of Natural Products (in Chinese), Beijing:People’s Medical Publishing House, 1993, 272.
Hu, C., Antioxidative property of flavonoid,China Oils and Fats (in Chinese), 1996, 21(4): 18.
Roginsky, V. A., Barsukova, T. K., Remorova, A. A. et al., Moderate antioxidative efficiencies of flavonoids during peroxidation of methyl linoleate in homogeneous and micellar solutions,J. Am. Oil Chem. Soc., 1996, 73(6): 777.
Van Acker, S. A. B. E., Koymans, L. M. H., Bast, A., Molecular pharmacology of vitamin E:structural aspects of antioxidant activity,Free Rad. Biol. Med., 1993, 15: 311.
Zhang, H. Y., Selection of theoretical parameter characterizing scavenging activity of antioxidants on free radicals,J. Am. OilChem. Soc., 1998. 75(12):in press.
Gajewski, J. J., Gilbert, K. E., Mckelvey, J.,MMX. an enhanced version of MM2, Adv. Mol. Model, 1990, 2: 65.
Dewar, M. J. S., Zoebisch, E. G., Healy, E. F. et al., AM1:a new general purpose quantum mechanical molecular model,J. Am. Chem. Soc., 1985, 107: 3902.
Hu, C., Ding, X. L., Antioxidant effect of flavonoid in different oxidation systems,Food and Fermentation Industries (in Chinese), 1996, 22(3): 46.
Ogata, M., Hoshi, M., Shimotohno, K. et al., Antioxidant activity of magnolol, honokiol, and related phenolic compounds,J. Am. Oil Chem. Soc., 1997, 74(5): 557.
Author information
Authors and Affiliations
Additional information
Project supported by the Doctoral Foundation of Science of Shandong Teachers University
Rights and permissions
About this article
Cite this article
Zhang, H. Theoretical elucidation of structure-activity relationship of flavonoid antioxidants. Sc. China Ser. B-Chem. 42, 106–112 (1999). https://doi.org/10.1007/BF02883044
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02883044