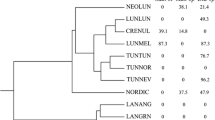
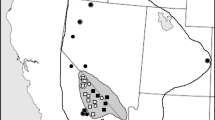
Abstract
South AmericanChenopodium assignable to sect.Chenopodium subsect.Cellulata (Chenopodiaceae) have been classified on the basis of fruit and leaf blade morphology. Samples representing 99 free-living and domesticated populations were included in a comparative study based on electrophoretic and morphometric data. The resulting patterns of variation indicate that past reliance on the fruit for diagnostic characters has obscured biological relationships. Domesticated and free-living populations of the high Andes, distributed from northwestern Argentina to Colombia, are closely allied and clearly separate from domesticated populations of coastal Chile and free-living populations of Argentina. Circumscription of the ArgentineC. hircinum to include Andean populations is rejected. Specific differentiation among Andean populations, polyphyletic origins forC. quinoa, and the presence of different ploidy levels are not indicated. Free-living Andean types sympatric withC. quinoa are provisionally placed within that species as subsp. milleanum. While the coastal quingua domesticate is clearly distinct from the Andean weed/crop complex, it is provisionally placed within subsp.quinoa to conserve established nomenclature. The overall pattern of morphogenetic variation among South American populations suggests a co-evolutionary relationship between domesticated and free-living populations of the high Andes, with a center of diversity at the southern extreme of the Andean range. Populations ofC. hircinum represent a logical link to the progenitor of the quinua complex, although firm phyletic and systematic alignments will require more information concerning populations of south-central Chile, and further definition of relative affinities among North and South American elements of subsectionCellulata.
Résumé
La biosistemática de la quinua II: Poblaciones indomesticadas. Las especies deChenopodium sudamericanas asignadas a la secciónChenopodium subsecciónCellulata se han clasificado en base a la morfolgía del fruto y de la hoja. Se hizo un estudio comparativo que incluyó 99 poblaciones de indomesticadas y domesticadas utilizando datos morfométricos y electroforéticos. Los patrones de variación resultantes indican que el uso de las características del fruto como diagnóstico ha oscurecido las relaciones biológicas existentes. Las poblaciones provenientes de los altos Andes entre el noroeste Argentino y Colombia son muy relacionados y claramente separados de las poblaciones domesticadas de Chile y las indomesticadas de Argentina. Se descarta la inclusión de las poblaciones andinas dentro de la especie argentinaC. hircinum. No se encuentra la evidencia para diferenciación específica dentro de las poblaciones andinas; para el origen polifilético deC. quinoa, y para la presencia de diferentes niveles deploidia. Los tipos andinos indomesticados simpatricos conC. quinoa se ubican provisionalmente en la subespeciemilleanum. Aunque la quingua costeña domesticada es claramente diferente del complejo andino maleza/cultivo se ubica provisionalmente en la subespecie quinoa para conservar la nomenclatura establecida. El patrón general de distribución morfogenética entre las poblaciones sudamericanas sugiere una relación coevolutiva entre poblaciones domesticadas y las indomesticadas de los Andes altos con un centro de diversidad en el extremo sur de la distribución en los Andes. Las poblaciones deC. hircinum representan una unión lógica al progenitos del complejo quinua aunque se necesitan mayor información sobre las poblaciones del centro-sur de Chile para tener evidencia filogenética y sistemática más firme. También es necesaria una definición de las afinidades relativas entre los elementos de Norte y Suramérica en la subsecciónCellulata.
Similar content being viewed by others
Literature Cited
Aellen, P. 1929. Beitrag zur Systematik derChenopodium-Arten Amerikas, vorweigend auf Grund der Sammlung des United States National Museum in Washington, D.C. Repert. Spec. Nov. Regni Veg. 26:31–64, 119–160.
—. 1960. Chenopodium. Pages 533–657in G. Hegi, ed., Illustrierte Flora von Mitteleuropa. 2nd ed. Vol. 3. C. Hanser, Munich.
—, and T. Just. 1943. Key and synopsis of the American species of the genusChenopodium L. Amer. Midl. Naturalist 30:47–67.
Brücher, H. 1987. The Isthmus of Panama as a crossroad for prehistoric migration of domesticated plants. Geojournal 14:121–122.
Cusack, D. F. 1984. Quinua: grain of the Incas. The Ecologist 14:21–31.
Gandarillas, H. 1984. Obtención experimental deChenopodium quinoa Willd. Instituto Boliviano de Tecnología Agropecuaria, La Paz.
Harlan, J. R. 1965. The possible role of weed races in the evolution of cultivated plants. Euphytica 14:177–188.
Herron, J. W. 1953. Study of seed production, seed identification, and seed germination ofChenopodium spp. Mem. New York Agric. Exp. Sta. 321:3–24.
Hunziker, A. T. 1952. Los pseudocereales de 1a agricultura indígena de América. ACME Agency, Buenos Aires.
Nei, M. 1978. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590.
Pimentel, R. A., and J. D. Smith. 1986. BIOSTAT II—a multivariate statistical toolbox. Sigma Soft, Placentia, CA.
Risi, J. C., and N. W. Galwey. 1984. TheChenopodium grains of the Andes: Inca crops for modern agriculture. Adv. Appl. Biol. 10:145–216.
Rogers, J. S. 1972. Measures of genetic similarity and genetic distance. Studies in Genetics. Univ. Texas Publ. 7213:145–153.
Sauer, C. O. 1969. Agricultural origins and dispersals. MIT Press, Cambridge, MA.
Simmonds, N. W. 1965. The grain chenopods of the tropical American highlands. Econ. Bot. 19: 223–235.
—. 1976. Quinoa and relatives. Pages 29–30in N. W. Simmonds, ed., Evolution of crop plants. Longman, New York.
Smith, B. D. 1984.Chenopodium as a prehistoric domesticate in eastern North America: evidence from Russell Cave, Alabama. Science 226:165–167.
—. 1985.Chenopodium berlandieri ssp.jonesianum: evidence for a Hopewellian domesticate from Ash Cave, Ohio. Southe. Archaeol. 4:107–133.
—, and V. A. Funk. 1985. A newly described subfossil cultivar of Chenopodium (Chenopodiaceae). Phytologia 57:445–448.
Swofford, D. L., and R. B. Selander. 1981. BIOSYS-1, a computer program for the analysis of allelic variation in genetics. User’s manual. Univ. Illinois, Urbana.
Tapia, M. 1979. Historia y distribución geográfica. Pages 11–19in M. Tapia, ed., Quinua y kañiwa: cultivos andinos. CIID, Bogota.
Walters, T. W. 1985. Analysis of systematic and phyletic relationships among alveolate-fruitedChenopodium of western North America. Ph.D. dissertation, Texas A&M University, College Station.
West, G. C. 1967. Nutrition of tree sparrows during winter in central Illinois. Ecology 48:58–67.
Williams, J. T., and J. L. Harper. 1965. Seed polymorphism and germination I. The influence of nitrates and low temperatures on the germination ofChenopodium album. Weed Res. 5:141–150.
Wilson, H. D. 1976. Genetic control and distribution of leucine aminopeptidase in the cultivated chenopods and related weed taxa. Biochem. Genet. 14:913–919.
—. 1980. Artificial hybridization among species ofChenopodium sectionChenopodium. Syst. Bot. 5:253–263.
—. 1981a. Genetic variation among tetraploidChenopodium populations of southern South America (sect.Chenopodium subsect.Cellulata). Syst. Bot. 6:380–398.
—. 1981b. DomesticatedChenopodium of the Ozark Bluff Dwellers. Econ. Bot. 35:233–239.
—. 1988a. Allozyme variation and morphological relationships ofChenopodium hircinum (s.l.). Syst. Bot. 13:215–228.
—. 1988b. Quinua biosystematics I: domesticated populations. Econ. Bot. 42:461–477.
—, and C. B. Heiser, Jr. 1979. The origin and evolutionary relationships of ‘Huauzontle’ (Chenopodium nuttalliae Safford), domesticated chenopod of Mexico. Amer. J. Bot. 66:198–206.
—, S. C. Barber, and T. W. Walters. 1983. Loss of duplicate gene expression in tetraploidChenopodium. Biochem. Syst. & Ecol. 11:7–13.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wilson, H.D. Quinua biosystematics II: Free-living populations. Econ Bot 42, 478–494 (1988). https://doi.org/10.1007/BF02862792
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02862792