Abstract
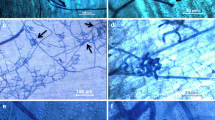
Development ofHelminthosporium solani Dur. & Mont. on artificially inoculated, excised potato tuber periderm, incubated in darkness and high humidity at 20 to 24°C, was studied using light and scanning electron microscopy. Spore germination occurred within 16 hours, and appressoria were observed 2 days after inoculation. Penetration of periderm was evident 4 days after inoculation. Conidiophores with young conidia developed on the periderm surface within 7 days of inoculation. Well-developed conidia were visible often by 9 days after inoculation. Rudimentary stromata were observed beneath conidiophore groupings at later stages of development.
In histological studies,H. solani hyphae were found in the phellem, phelloderm, and cortex of infected tuber sections. Basal cells of conidiophores or stromata were observed on the surface and in the outer tangential cork cell layers. Several layers of suberized and sometimes collapsed cortical cells were observed beneath disrupted and collapsed, infected periderm. Cavities sometimes formed between periderm and cortex, and callosities were observed on cell walls of some cortical cells beneath infected periderm.
Resumen
Con microscopía de luz y de exploración electrónica se estudió el desarrollo deHelminthosporium solani Dur. & Mont. sobre peridermis extirpada de tubérculos de papa, artificialmente inoculadas e incubada bajo condiciones de obscuridad, alta humedad y 20 a 24°C. La germinación de esporas tuvo lugar alrededor de 16 horas después de la inoculación. A los dos días de la inoculación se observaron apresorios. La penetración en la peridermis fue evidente cuatro dias después de la inoculación, y a los siete días se desarrollaron conidióforos con conidias jóvenes sobre la superficie de la peridermis. Se vieron conidias bien desarrolladas comúnmente nueve días después de inocular el patógeno. Se observaron estromas rudimentarios debajo de los grupos de conidióforos en estadíos posteriores de desarrollo.
En estudios histológicos, fueron detectadas hijas deH. solani en la peridermis, la felodermis y la cortex de secciones infectadas de tubérculos. Se observaron células basales de conidióforos o estromas sobre la superficie y en las capas corchosas externas y tangenciales (epidermis). Se observó suberización y a veces colapso de varias capas de células corticales por debajo de la peridermis infectada, desorganizada y flácida. Se formaron a veces cavidades entre la peridermis y la cortex, y se observaron callosidades sobre las paredes celulares de algunas células corticales debajo de la peridermis infectada.
Similar content being viewed by others
Literature Cited
Akai, S. 1959. Histology of defense in plants. Pp 391–434In Plant Pathology: an Advanced Treatise, Vol. 1: The Diseased Plant. J.G. Horsfall and A.E. Dimond, eds. Academic Press, New York. 674 pp.
Artschwager, E. 1927. Wound periderm formation in the potato as affected by temperature and humidity. J Agric Res 35(11):995–1000.
Burke, O.D. 1938. The silver-scurf disease of potatoes. Cornell Univ Agric Exp Stn Bull 692. 30 pp.
Bushneil, W.R. 1972. Physiology of fungal haustoria. Annu Rev Phytopathol 10:151–176.
Clark, G., ed. 1973. Staining Procedures used by the Biological Stain Commission. Published for the Biological Stain Commission. The Williams & Wilkins Co., Baltimore. 418 pp.
Esau, K. 1953. Plant Anatomy. John Wiley & Sons, Inc., New York. 735 pp.
Fellows, H. 1928. Some chemical and morphological phenomena attending infection of the wheat plant byOphiobolus graminis. J Agric Res 37(11):647–661.
Hawkins, L.A. and R.B. Harvey. 1919. Physiological study of the parasitism ofPythium debaryanum Hesse on the potato tuber. J Agric Res 18(5):275–297.
Heiny, D.D.K. 1982.Helminthosporium solani development and silver scurf or black dot incidence on potato periderm. M.S. Thesis, Dept. of Botany and Plant Pathology. Colorado State University, Fort Collins, Colorado. 151 pp.
Heiny, D.K. and G.A. McIntyre. 1981. The development ofHelminthosporium solani on potato tuber periderm. Phytopathology 71:1004. (Abstract).
Hohl, H.R. and P. Stossel. 1976. Host-parasite interfaces in a resistant and a susceptible cultivar ofSolanum tuberosum inoculated withPhytophthora infestons: tuber tissue. Can J Bot 54:900–912.
Hunger, R.M. 1978. Occurrence, development, and losses associated with silver scurf and black dot on Colorado potatoes. M.S. Thesis, Dept. of Botany and Plant Pathology. Colorado State University, Fort Collins, Colorado. 81 pp.
Hunger, R.M. and G.A. McIntyre. 1979. Occurrence, development, and losses associated with silver scurf and black dot on Colorado potatoes. Am Potato J 56:289–306.
Ito, K. 1949. Studies on “Murasaki-Mompa” disease caused byHelicobasidium mompa Tanaka. Bull Gov For Exp Stn Tokyo 43:1–126.
Jellis, G.J. 1972. Silver scurf disease of potatoes. Ph.D. Thesis. University of Manchester.
Jellis, G.J. and G.S. Taylor. 1974. The relative importance of silver scurf and black dot: two disfiguring diseases of potato tubers. A.D.A.S. (Agric. Dev. Advis. Serv.) Q Rev 14:53–61.
Jellis, G.J. and G.S. Taylor. 1977. The development of silver scurf (Helminthosporium solani) disease of potato. Ann Appl Biol 86:19–28.
Johansen, D.A. 1940. Plant Microtechnique. McGraw-Hill Book Co., Inc., New York. 523 pp.
Lennard, J.H. 1968. Experimental work. The Edinburgh School of Agriculture. Rev Plant Pathol (1970) 49:44–45. (Abstract).
Luttrell, E.S. 1963. Taxonomic criteria inHelminthosporium. Mycologia 55:643–674.
Mooi, J.C. 1968. De aantasting van de aardappel door zilver-schurft (Helminthosporium solani). Versl Landbouwkd Onderz (Agric. Res. Rep.) 716. 62 pp.
Murray, G.M. and D.P. Maxwell. 1975. Penetration ofZea mays byHelminthosporium carbonum. Can J Bot 53:2872–2883.
Nisikado, Y. 1928. Studies on theHelminthosporium diseases of Gramineae in Japan. Ohara Inst Agric Res Special Rep 4:1–394. (in Japanese, English descriptions: English summary in Ber Ohara Inst Landwirtsch Forschung Kurashiki 4:111–126. 1929).
Nnodu, E.C. 1980. The effect of temperature and humidity on the infection of potato tubers byAlternaria solani. Ph.D. Thesis, Dept. of Botany and Plant Pathology. Colorado State University, Fort Collins, Colorado. 99 pp.
Priestley, J.H. and L.M. Woffenden. 1923. The healing of wounds in potato tubers and their propagation by cut sets. Ann Appl Biol 10:96–115.
Sass, J.E. 1958. Botanical Microtechnique. 3rd ed. The Iowa State University Press, Ames, Iowa. 228 pp.
Schultz, E.S. 1916. Silver-scurf of the Irish potato caused bySpondylocladium atrovirens. J Agric Res 6(10):339–350.
Simmons, S.A. and R.A. Shoemaker. 1952. Differential staining of fungus and host cells using a modification of Pianese IIIb. Stain Technol 27:121.
Steele, C.C. 1934. An Introduction to Plant Biochemistry. G. Bell & Sons, Ltd., London. 356 pp.
Stevens, R.B., ed. 1974. Mycology Guidebook. University of Washington Press, Seattle. 703 pp.
Taubenhaus, J.J. 1916. A contribution to our knowledge of silver scurf (Spondylocladium atrovirens Harz) of the white potato. Mem NY Bot Gard 6:549–560.
Wenzl, H. 1969. The decomposition of starch in potato tubers byH. solani (silver scurf). Rev Plant Pathol (1971) 50:48–49. (Abstract).
Young, P.A. 1926. Penetration phenomena and facultative parasitism inAlternaria, Diplodia, and other fungi. Bot Gaz 81:258–278.
Author information
Authors and Affiliations
Additional information
From a thesis submitted to the Academic Faculty of Colorado State University in partial fulfillment of the requirements for the degree of Master of Science.
Former Graduate Research Assistant, Department of Botany and Plant Pathology, Colorado State University, Fort Collins, Colorado 80523.
Rights and permissions
About this article
Cite this article
Heiny, D.K., Mclntyre, G.A. Helminthosporium solani Dur. & Mont. development on potato periderm. American Potato Journal 60, 773–789 (1983). https://doi.org/10.1007/BF02856896
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02856896