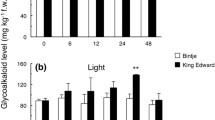
Abstract
The steroid glycoalkaloids are triterpenoid derivatives which are found in all tissues of the potato plant including the tubers. The compounds are largely localized in the peel of tubers, but tissue beneath the peel rapidly accumulates the steroid glycoalkaloids to levels equal to or greater than those in the peel as a result of injury or environmental stress. The accumulation is restricted to the outer 1–2 mm of injured or stressed tuber.
Potatoes containing over 0.02% steroid glycoalkaloids are considered toxic to man, and at this concentration they would impart a distinctly bitter flavor. The accumulation of steroid glycoalkaloids is suppressed and the accumulation of sesquiterpenoids is elicited in tubers infected by various pathogens and nonpathogens including the late blight pathogen,Phytophthora infestans. Arachidonic acid and eicosapentaenoic acids, two polyunsaturated fatty acids isolated fromP. infestans, are potent inhibitors of steroid glycoalkaloid accumulation. Both acids elicit the localized accumulation of sesquiterpenoids including rishitin, lubimin, phytuberin, phytuberol and solavetivone. Rishitin and lubimin generally comprise 85–90% of the total sesquiterpenoids which accumulate. The steroid glycoalkaloids and sesquiterpenoids appear to have a role in disease resistance to some fungal pathogens. Both groups of compounds are synthesized via the acetate-mevalonate pathway. Arachidonic and eicosapentaenoic acids appear to inhibit steroid glycoalkaloid accumulation at the level of the conversion of farnesyl pyrophosphate to squalene and they activate the biosynthesis of sesquiterpenoids. The reduction of steroid glycoalkaloids in potato foliage and tubers for health and flavor considerations should be considered relative to the ability of tubers and foliage to accumulate sesquiterpenoids in response to infection and its influence on disease and insect resistance.
Resumen
Los glicoalcaloides esteroideos son derivados triterpenoides que se encuentran en todos los tejidos de la planta de papa, incluyendo los tubérculos. Los compuestos están sobre todo ubicados en la cáscara de los tubérculos, pero el tejido debajo de la cáscara acumula rápidamente los glicoalcaloides esteroideos, llegando éstos a niveles iguales o superiores a los de la cáscara como resultado de algun lesion o estrés ambiental. La acumulación se limita a un espesor de 1–2 mm en la parte exterior del tubérculo lesionado o que haya sufrido el estrés.
La papa que contiene más de 0,02% de glicoalcaloides esteroideos, es considerada tóxica para el ser humano. Cuando se encuentran en esta concentración, la papa hace sentir un sabor netamente amargo. Se suprime la acumulación de glicoalcaloides esteroideos y se produce la acumulación de sesquiterpenoides en los tubérculos infectados de diferentes patógenos y no patógenos, incluyendo el patógeno del tizón tardio,Phytophthora infestons. El ácido araquidónico y el ácido eicosapentaenoico, dos ácidos grasos poliinsaturados que han sido aislados deP. infestans, son inhibidores potentes de la acumulación de alcaloides esteroideos. Los dos ácidos producen la acumulación localizada de sesquiterpenoides, incluyendo risitina, lubimina, fituberina, fituberol y solavetivona. Generalmente, la risitina y la lubimina constituyen 85%–90% del total de sesquiterpenoides que se acumulan. Los glicoalcaloides esteroideos y los sesquiterpenoides parecen jugar un papel en la resistencia a enfermedades causadas por algunos patógenos fungosos. Ambos grupos de compuestos se sintetizan por vía de mevalonato de acetato. Los ácidos araquidónico y eicosapentaenoico parecen inhibir la acumulación de glicoalcaloides esteroides al nivel de la conversión del pirofosfato de farnesil en escualeno y activan la biosíntesis de los sesquiter-penoides. La reducción de los glicoalcaloides esteroideos en el follaje y los tubérculos de la papa, desde un punto de vista de salubridad y sabor, debería ser considerada en relación con la capacidad de los tubérculos y del follaje para acumular sesquiterpenoides en respuesta a infecciones y en relación con su influencia sobre la resistencia a enfermedades e insectos.
Similar content being viewed by others
Literature Cited
Allen, E. and J. Kuc. 1968. α-Solanine and α-chaconine as fungitoxic compounds in extracts of Irish potato tubers. Phytopathology 58:776–781.
Allen, R.J., R.J. Marlar, G.F. Chesney, J.P. Helgeson, A. Kelman, K.G. Weckel, E. Traisman and J.W. White. 1977. Teratogenicity studies on late blighted potatoes in nonhuman primates (Macaca mulatto andSaguinis labiatus). Teratology 15:17–24.
Alves, L.M. 1980. Regulation of stress metabolite biosynthesis in potato tuber during hypersensitivity. Phytopathology 70:458.
Alves, L.M., E.G. Heisler, J.C. Kissinger, J.M. Patterson and E.B. Kalan. 1979. Effects of controlled atmospheres on production of sesquiterpenoid stress metabolites by white potato tuber. Plant Physiol 63:359–362.
Bostock, R.M. 1981. “The Identification of Polyunsaturated Fatty Acids from Phytophthora Infestans as Elicitors of Hypersensitivity in Potato Tuber and Studies of Related Terpenoid Metabolism”. Ph.D. Thesis Univ. of Kentucky, Lexington, Kentucky USA.
Bostock, R.M., J. Kuc and R.A. Laine. 1981. Eicosapentaenoic and arachidonic acids fromPhytophthora infestans elicit fungitoxic sesquiterpenes in potato. Science 212: 67–69.
Bostock, R.M., R.A. Laine and J. Kuc. 1981. Enhanced elicitor activity of eicosapentaenoic and arachidonic acids by factors in the mycelium ofPhytophthora infestans. Phytopathology 71:861.
Cheema, A.S. and N.F. Haard. 1978. Induction of rishitin and lubimin in potato tuber discs by non-specific elicitors and the influence of storage conditions. Physiol Plant Pathol 13:233–240.
Currier, W.W. and J. Kuc. 1975. Effect of temperature on rishitin and steroid glycoalkaloid accumulation in potato tuber. Phytopathology 65:1194–1197.
Doke, N. 1975. Prevention of the hypersensitive reaction of potato cells to infection with incompatible races ofPhytophthora infestans by constituents of zoospores. Physiol Plant Pathol 7:1–7.
Doke, N.A., N.A. Garas, and J. Kuc. 1979. Partial characterization and aspects of the mode of action of a hypersensitive-inhibiting factor (HIF) isolated fromPhytophthora infestans. Physiol Plant Pathol 15:127–140.
Doke, N., N. Garas and J. Kuc. 1980. Effect on host hypersensitivity of suppressors released during the germination of cystospores ofPhytophthora infestans. Phytopathology 70:35–39.
Doke, N. and K. Tomiyama. 1980. Effect of hyphal wall components fromPhytophthora infestans on protoplasts of potato tuber tissues. Physiol Plant Pathol 16:169–176.
Doke, N. and K. Tomiyama. 1980. Suppression of the hypersensitive response of potato tuber protoplasts to hyphal wall components by water soluble glucans isolated fromPhytophthora infestans. Physiol Plant Pathol 16:177–186.
Garas, N.A., N. Doke and J. Kuc. 1979. Suppression of the hypersensitive reaction in potato tubers by mycelial components fromPhytophthora infestans. Physiol Plant Pathol 15:117–126.
Gregory, P. and S.L. Sinden. 1980. Glycoalkaloids of some wild potato varieties differing in insect resistance. Am Potato J 57:478.
Guseva, A.R. and V.A. Paseshnichenko. 1957. Enzymic degradation of potato glycoalkaloids. Biochemistry USSR 22:782–799.
Guseva, A.R., V.A. Paseshnichenko and M.G. Borikhina. 1961. Synthesis of radioactive mevalonic acid and its use in the study of the biosynthesis of steroid glycoalkaloids fromSolanum. Biochemistry USSR 26:631–635.
Harris, J.E. and C. Dennis. 1976. Antifungal activity of postinfectional metabolites from potato tubers. Physiol Plant Pathol 9:155–165.
Harris, J.E. and C. Dennis. 1977. The effect of post-infectional potato tuber metabolites and surfactants on zoospores of Oomycetes. Physiol Plant Pathol 11:163–169.
Henfling, J.W.D.M., N. Lisker and J. Kuc. 1978. Effect of ethylene on phytuberin and phytuberol accumulation in potato tuber slices. Phytopathology 68:857–862.
Henfling, J.W.D.M., R.M. Bostock and J. Kuc. 1980. Cell walls ofPhytophthora infestans contain an elicitor of terpene accumulation in potato tubers. Phytopathology 70:772–776.
Henfling, J., R.M. Bostock and J. Kuc. 1981. Effect of abscisic acid on rishitin and lubimin accumulation and resistance toPhytophthora infestans andCladosporium cucumerinum in potato tuber tissue slices. Phytopathology 70:1074–1078.
Holland, H.L. and G.J. Taylor. 1979. Transformations of steroids and the steroidal alkaloid, solanine byPhytophthora infestans. Phytochemistry 18:437–440.
Horikawa, T., K. Tomiyama and N. Doke. 1976. Accumulation and transformation of rishitin and lubimin in potato tuber tissue infected by an incompatible race ofPhytophthora infestans. Phytopathology 66:11186–1191.
Ishiguri, Y., K. Tomiyama, N. Doke, A. Murai, N. Katsui, F. Yagihashi and T. Masamune. 1978. Induction of rishitin-metabolizing activity in potato disks by wounding and identification of rishitin metabolites. Phytopathology 68:720–725.
Ishiguri, Y., K. Tomiyama, A. Murai, N. Katsui and T. Masamune. 1978. Toxicity of rishitin, rishitin-M-1 and rishitin-M-2 toPhytophthora infestans and potato tissue. Ann Phytopathol Soc Jpn 44:52–56.
Ishizaki, N. and K. Tomiyama. 1972. Effect of wounding or infection byPhytophthora infestans on the content of terpenoids in potato tubers. Plant and Cell Physiol 13:1053–1063.
Jakhav, S.J., D.K. Salunkhe, R. Wyse and R.R. Dalvi. 1973. Solanum alkaloids; biosynthesis and inhibition of chemical treatments. J Food Sci 38:453–455.
Jadhav, S.J. and D.K. Salunkhe. 1975. Formation and control of chlorophyll and solanine in tubers ofSolanum tuberosum L. and evaluation of solanine toxicity. Adv Food Res 21:307–354.
Johnson, D.F., R.D. Benet and E. Heftman. 1963. Cholesterol in higher plants. Science 140:198.
Kuc, J. 1972. Compounds accumulating in plants after infection.In “Microbial Toxins” (Ajl, S., Weinbaum, G. and Kadis, S. eds.) Vol. 8 pp. 211–247. Academic Press, N.Y.
Kuc, J. 1975. Teratogenic constituents of potatoes. Recent Adv Phytochem 9:139–150.
Kuc, J. 1976. Phytoalexins, plants and human health.In “Mycotoxins and Other Fungal-Related Food Problems”. (J. Rodricks, ed.) pp. 356–368. Am Chem Soc, Washington, D.C.
Kuc, J. 1982. Phytoalexins from the Solanacae.In “Phytoalexins” (Bailey, J. and Mansfield, J., eds.) pp. 81–105. Blackie, Glasgow, London.
Kuc, J., W.W. Currier and M.J. Shih. 1976. Terpenoid phytoalexins.In “Biochemical Aspects of Plant-Parasite Relationships” (Friend, J. and Threlfall, D.R., eds.) pp. 225–237. Academic Press, London, N.Y.
Kuc, J. and L. Shain. 1977. Antifungal compounds associated with disease resistance in plants.In “Antifungal Compounds,” Vol. 2 (Siegel, M. and H.D. Sisler, eds.) pp. 497–535. Marcel Dekker, N.Y., Basel.
Kuc, J. and N. Lisker. 1978. Terpenoids and their role in wounded and infected plant storage tissues.In “Biochemistry of Wounded Plant Tissues” (Kahl, G., ed.) pp. 203–242. Walter de Gruyter, Berlin, N.Y.
Kuc, J., J. Henfling, N. Garas and N. Doke. 1979. Control of terpenoid metabolism in the potato —Phytophthora infestons interaction. J Food Prot 42:508–511.
Kurantz, M.J. and Zacharius, R.M. (1981). Hypersensitive response in potato tuber: elicitation by combination of non-eliciting components fromPhytophthora infestons. Physiol Plant Pathol 18:67–77.
Lisker, N. and J. Kuc. 1977. Elicitors of terpenoid accumulation in potato tuber slices. Phytopathology 67:1356–1359.
Locci, R. and J. Kuc. 1967. Steroid glycoalkaloids as compounds produced by potato tubers under stress. Phytopathology 57:1272–1273.
Malmberg, A.G. and Theander, O. 1980. Two phytoalexin glycosides from potato tubers infected withPhoma. Phytochemistry 19:1739–1742.
Maniara, G. and J. Kuc. 1983. Manuscript accepted for Physiol Plant Pathology.
McCay, CM., J.B. McCay and O. Smith. 1975. The nutritive value of potatoes.In “Potato Processing” (W.F. Talburt and O. Smith, eds.) pp. 235–273. Avi Publishing Co., Westport, Connecticut.
McKee, R. 1955. Host-parasite relationship in the dry-rot disease of potatoes. Ann Appl Biol. 43:147–148.
McKee, R. 1959. Factors affecting the toxicity of solanine and related alkaloids toFusarium caeruleum. J. Gen Microbiol 20:686–696.
McMillan, M. and J.C. Thompson. 1979. An outbreak of suspected solanine poisoning in school boys. Quart J Med 48:227–248.
Paseshnichenko, V.A. 1957. Content of solanine and chaconine in the potato during the vegetation period. Biochemistry USSR 22:929–931.
Patil, B.C., D.K. Salunkhe and B. Singh. 1971. Metabolism of solanine and chlorophyll in potato tubers as affected by light and chemicals. J Food Sci 36:474–476.
Price, K.R., B. Howard and D.T. Coxon. 1976. Stress metabolite production in potato tubers infected byPhytophthora infestons, Fusarium avenaceum andPhoma exigua. Physiol Plant Pathol 9:189–197.
Renwick, J.H. 1972. Hypothesis: Anencephaly and spina bifida are usually preventable by avoidance of a specific but unidentified substance present in certain potato tubers. Br J Prev Soc Med 26:67–88.
Renwick, J. 1972. Prevention of anencephaly and spina bifida in man. Teratology 8:321–323.
Ripperger, H., W. Moritz and K. Schreiber. 1971. Zur biosynthese von solanum-alkaloiden aus cycloartenol oder lanosterol. Phytochemistry 10:2699–2704.
Salunkhe, D.K. and T.M. Wu. 1979. Control of postharvest glycoalkaloid formation in potato tubers. J Food Prot 42:519–525.
Sapeika, N. 1969. Foods of plant origin.In “Food Pharmacology” Thomas Publishing Co., Springfield, IL.
Sato, N., K. Tomiyama, N. Katsu and T. Masamune. 1968. Isolation of rishitin from tubers of interspecific potato varieties containing different late blight genes. Ann Phytopathol Soc Jpn 34:140–142.
Schreiber, J. 1968. Steroid Alkaloids: The Solanum Group.In “The Alkaloids Chemistry and Physiology”. Vol. 10 (Manske, R.H.F., ed.) pp. 1–192. Academic Press, N.Y.
Shih, M. 1972. “The Accumulation of Isoprenoids and Phenols and its Control as Related to the Interaction of Potato (Solanum tuberosum) withPhytophthora infestons.” Ph.D. Thesis, Purdue Univ., Lafayette, Indiana USA.
Shih, M.J., J. Kuc and E.B. Williams. 1973. Suppression of steroid glycoalkaloid accumulation as related to rishitin accumulation in potato tubers. Phytopathology 63:821–826.
Shih, M.J. and J. Kuc. 1973. Incorporation of14C from acetate and mevalonate into rishitin and steroid glycoalkaloids by potato slices inoculated withPhytophthora infestons. Phytopathology 63:826–829.
Shih, M.J. and J. Kuc. 1974. α- and β-Solamarine in KennebecSolanum tuberosum leaves and aged tuber slices. Phytochemistry 13:997–1000.
Sinden, S.L., L.L. Sanford and S.F. Osman. 1980. Glycoalkaloids and resistance to Colorado potato beetle inSolanum chacoense Bitter. Am Potato J 57:331–343.
Stoessl, A. 1982. Biosynthesis of phytoalexins.In “Phytoalexins” (Bailey, J. and Mansfield, J., eds.) pp. 133–180. Blackie, Glasgow, London.
Stoessl, A., J.B. Stothers and E.W.B. Ward. 1976. Sesquiterpenoid stress compounds of the Solanaceae. Phytochemistry 15:855–872.
Stoessl, A., E.W.B. Ward and J.B. Stothers. 1977. Biosynthetic relationships of sesquiterpenoidal stress compounds from the Solanaceae.In “Host Plant Resistance to Pests” (Hedin, P.A., ed.) pp. 61–77. Am Chem Soc, Washington, D.C.
Stoessl, A., J.B. Stothers and E.W.B. Ward. 1978., Biosynthetic studies of stress metabolites from potatoes: incorporation of sodium acetate —13C2 into 10 sesquiterpenes. Can J Chem 56:645–653.
Subramanian, S., J.L. Varns and J. Kuc. 1971. Biochemical response in the compatible potato tuber—Phytophthorainfestons interaction. Proc Indiana Acad Sci 80:367.
Swarze, P. 1962. Methods for identification and determination of solanine in potato breeding material. Zuchter 32:155–160.
Tingey, W.M., J.D. Mackenzie and P.F. Gregory. 1978. Total foliar glycoalkaloids and resistance of wild potato species toEmpoasca fabae (Harris). Am Potato J 55:577–585.
Tjamos, E. and J. Kuc. 1982. Inhibition of steroid glycoalkaloid accumulation by arachidonic and eicosapentaenoic acids in potato. Science 217:542–544.
Tjamos, E., E. Nuckles and J. Kuc. 1983. Regulation of steroid glycoalkaloid and sesquiterpenoid stress metabolite accumulation in potato tubers by inhibitors of steroid synthesis and phytohormones. Submitted to Physiol Plant Pathol.
Tjamos, E., M. Siegel and J. Kuc. 1983. Fungitoxicity of polyunsaturated fatty acids. Prepared for publication. Phytopathology.
Tomiyama, J. 1966. Double infection by an incompatible race ofPhytophthora infestons of potato cell which has previously been infected by a compatible race. Ann Phytopathol Soc Jpn 32:181–185.
VanEtten, H., D.E. Mathews and D.A. Smith. 1982. Metabolism of phytoalexins.In “Phytoalexins” (Bailey, J. and Mansfield, J., eds.) pp. 181–217. Blackie, Glasgow, London.
Varns, J. 1970. “Biochemical Response and its Control in the Irish Potato Tuber (Solanum tuberosum)—Phytophthorainfestons Interaction”. Ph.D. Thesis, Purdue Univ., Lafayette, Indiana, USA.
Varns, J., J. Kuc and E. Williams. 1971. Terpenoid accumulation as a biochemical response of the potato tuber toPhytophthora infestons. Phytopathology 61:174–177.
Varns, J. and J. Kuc. 1971. Suppression of rishitin and phytuberin accumulation and hypersensitive response in potato by compatible races ofPhytophthora infestons. Phytopathology 61:178–181.
Varns, J., W.W. Currier and J. Kuc. 1971. Specificity of rishitin and phytuberin accumulation by potato. Phytopathology 61:968–971.
Varns, J. and J. Kuc. 1972. Suppression of the resistance response as an active mechanism for susceptibility in thepotato-Phytophthora infestons interaction.In “Phytotoxins in Plant Diseases” (Wood, R.K.S. and A. Graniti, eds.) pp. 465–469. Academic Press, London, N.Y.
Ward, E.W.B. and A. Stoessl. 1977. Phytoalexins from potatoes: evidence for the conversions of lubimin to 15-dihydrolubimin by fungi. Phytopathology 67:468–471.
Wilmont, S.C. 1933. An investigation of solanine poisoning. Analyst 58:431–439.
Wood, G. 1976. Stress metabolites of white potatoes.In “Mycotoxins and Other Fungal Related Food Problems” (Rodricks, J., ed.) pp. 369–386. Amer Chem Soc, Washington, D.C.
Wu, M.T. and D.K. Salunkhe. 1972. A research note. Control of chlorophyll and solanine syntheses and sprouting of potato tubers by hot paraffin wax. J Food Sci 37:629–630.
Wu, M.T. and D.K. Salunkhe. 1972. Inhibition of chlorophyll and solanine formation and sprouting of potato tubers by oil dipping. J Am Soc Hortic Sci 97(5):614–616.
Wu, M.T. and D.K. Salunkhe. 1977. Effect of gamma-irradiation on wound induced glycoalkaloid formation in potato tubers. Lebensm Wiss Technol 10:141–144.
Wu, M.T. and D.K. Salunkhe. 1977. Inhibition of wound induced glycoalkaloid formation in potato tubers (Solanum tuberosum L.) by isopropyl-N(3-chlorophenyl)-carbamate. J Food Sci 42:622–624.
Wu, M.T. and D.K. Salunkhe. 1978. After effect of anoxia water treatment on greening and glycoalkaloid formation of potato tubers. J Food Sci 43:1330–1331.
Zacharius, R.M., E.B. Kalan, S.F. Osman and S.F. Herb. 1975. Solanidine in potato (Solanum tuberosum) tuber tissue disrupted byErwinia atroseptica and byPhytophthora infestons. Physiol Plant Pathol 6:301–305.
Zacharius, R.M., S.F. Osman, E.G. Heisler and J.C. Kissinger. 1976. Effect of the R-3 gene in resistance of the Wauseon potato tuber toPhytophthora infestons. Phytopathology 66:964–966.
Zitnak, A. and G.R. Johnston. 1970. Glycoalkaloid content of B5141-6 potatoes. Am Potato J 47:256–260.
Author information
Authors and Affiliations
Additional information
Journal paper number 82-11-220 of the Kentucky Agricultural Experiment Station, Lexington, Kentucky 40546.
Rights and permissions
About this article
Cite this article
Kuc, J. Steroid glycoalkaloids and related compounds as potato quality factors. American Potato Journal 61, 123–139 (1984). https://doi.org/10.1007/BF02854034
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02854034