Summary
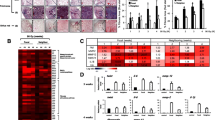
To investigate the effects of TGF-β1 on the gelatinases (MMP-2 and MMP-9), and their roles in lung remodeling after irradiation-induced lung injury. Expressions of TGF-β1 were measured with western blot, and expressions of MMP-2 and MMP-9 were analyzed with zymography in a TGF-β1 transgenic mouse model after thoracic irradiation with 12 Gy. We found expressions of TGF-β1 in the lung from the transgenic mice were three folds as compared to those from control mice. With densitometrical analysis, we found a significant decrease in MMP-9 activity in lung homogenates from the transgenic mice as compared with those from non-transgenic control mice 8 weeks after sham-irradiation (relative MMP-9 activity: C: 1.000±0.1091; TG: 0.4772±0.470 (n=8,P<0.05). But MMP-2 was constitutively expressed in the lung homogenates from the transgenic mice as compared to those from control mice 8 weeks after sham-irradiation (relative MMP-2 activity 8 weeks after sham-irradiation: C: 1.000±0.1556, TG: 1.0075±0.1472). Eight weeks after thoracic irradiation with 12 Gy, we observed a significant increase of MMP-2 and MMP-9 activity in lung homogenates from both transgenic and normal mice. In TGF-β1 transgenic mice relative MMP-9 activity was increased to 1.5321±0.2217 folds 8 weeks after thoracic irradiation with 12 Gy as compared to those after sham-irradiation (1.000±0.2153), and relative MMP-2 activity was increased to 1.7142±0.4231 folds. Our results show that TGF-β1 itself down-regulates activity of MMP-9, thereby decreases ECM degradation in lungs of TGF-β1 transgenic mice. Also we find that ionizing irradiation upregulates both MMP-2 and MMP-9 activity. Over-expressions of MMP-9 and MMP-2 after lung irradiation are involved in the inflammatory response associated with radiation-induced lung injury, and maybe further in radiation-induced lung fibrosis.
Similar content being viewed by others
References
Ruebe C E, Uthe D, Schmid K Wet al. Dose-dependent induction of transforming growth factorβin the lung tissue of fibrosis-prone mice after thoracic irradiation. Int J Radiation Oncology Biol Phys, 2000, 47:1033–1042
Sanderson N, Factor V, Nagy Pet al. Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci USA, 1995, 92:572–576
Smith P K, Krohn R I, Hermanson G Tet al. Measurement of protein using bicinchoninic acid. Anal Biochem, 1985, 150:76–85
Seeland U, Haeuseler C, Hinrichs Ret al. Myocardial fibrosis in transforming growth factor β1 (TGF-β1) transgenic mice is associated with inhibition of interstitial collagenase. European J of Clinical Investigation, 2002, 32:295–303
Faehlig M, Perlewitz A, Doller Aet al. Regulation of collagen prolyl 4-hydroxylase and matrix metalloproteinases in fibrosarcoma cells by hypoxia. Comp Biochem Physiol C: Toxicol Pharmacol, 2004, 139:119–126
Tanaka H, Hojo K, Yoshida Het al. Molecular cloning and expression of the mouse 105-kDa gelatinase cDNA. Biochem Biophys Res Commun, 1993, 190:732–740
Vaday G G, Schor H, Rahat M Aet al. Transforming growth factor-beta suppress tumor necrosis factor alpha-induced matrix metalloproteinase-9 expression in monocytes. J Leukoc Biol, 2001, 69:613–621
Zechel J, Gohil H, Lust W Det al. Alterations in matrix metalloproteinase-9 levels and tissue inhibitor of matrix metalloproteinases-1 expression in a transforming growth factor-β transgenic model of hydrocephalus. J Neurosci Res, 2002, 69:662–668
Corbel M, Lanchou J, Germain Net al. Modulation of airway remodeling-associated mediators by antifibrotic compound, pirfenidone, and the matrix metalloproteinase inhibitor, batimastat, during acute lung injury in mice. Eur J Pharmacol, 2001, 426:113–121
Hayashi T, Stetler-Stevenson W G, Fleming M Vet al. WD Immunoshistochemical study of metalloproteinases and their tissue inhibitors in the lungs of patients with diffuse alveolar damage and idiopathic pulmonary fibrosis. Am J Pathol, 1996, 149:1241–1256
Delclaux C, d’Ortho M P, Delacourt Cet al. Gelatinases in epithelial lining fluid of patients with adult respiratory distress syndr ome. Am J Physiol Lung Cell Mol Physiol, 1997, 272:L442-L451
Author information
Authors and Affiliations
Corresponding author
Additional information
YANG Kunyu, male, born in 1971, M. D., Ph. D.
Rights and permissions
About this article
Cite this article
Kunyu, Y., Li, L., Tao, Z. et al. TGF-beta1 transgenic mouse model of thoracic irradiation: Modulation of MMP-2 and MMP-9 in the lung tissue. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 26, 301–304 (2006). https://doi.org/10.1007/BF02829557
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02829557