Abstract
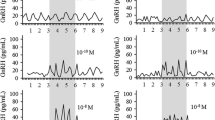
We have previously shown that the gonadal and neurosteroid, 3α-hydroxy-4-pregnen-20-one (3αHP), can selectively suppress gonadotrophin-releasing hormone (GnRH) induced follicle-stimulating hormone (FSH) release from static cultures of anterior pituitary cells during a 4-h incubation period. The actions appeared to be at the level of the gonadotroph membrane and the cell signaling pathway involving Ca2+ and protein kinase C (PKC). In order to investigate further if the effects of 3αHP on FSH release are generated by nongenomic mechanisms, we monitored the short-term effects of 3αHP using dispersed anterior pituitary cells in a low dead-volume perifusion system with short (≤5 min) exposures to the steroid. Pulses of GnRH (10−8 or 10−7 M) lasting 2–5 min resulted in marked peaks of FSH release, and the variation in FSH amounts released from the cells in a particular column were minimal if the interval between successive GnRH pulses was at least 3–4 h. A 5-min pulse of 3αHP (10−9 M) administered simultaneously with the GnRH pulse suppressed GnRH-induced FSH release. On the other hand, similar treatment with the stereoisomer 3β-hydroxy-4-pregnen-20-one (3βHP), had no effect, but progesterone and estradiol pulses augmented the GnRH-induced FSH release. Pretreatment of cells with a 5-min pulse of 3αHP, at 120, 60, or 30 min prior to a GnRH pulse suppressed the GnRH-induced FSH release. The suppression of GnRH-induced FSH release by 3αHP was only partial if the start of the 3αHP pulse occurred 0.5 or 1.0 min after the start of the GnRH pulse, and no suppression occurred if the start of the 3αHP pulse was delayed by 2–5 min. The FSH release elicited by 5-min pulses of the Ca2+ ionophore A23187, the Ca2+ agonist BAYK8644, the PKC activator phorbol 12-myristate 13-acetate (PMA), or phospholipase C (PLC) was suppressed by simultaneous pulses of 3αHP. The suppression of FSH release by 3αHP appeared to bestereospecific, since no suppression was observed with 5α-pregnane-3,20-dione (5αP) or 3α-hydroxy-5α-pregnan-20-one (5αP3α). In separate experiments, cells were treated with pulses of BSA conjugates of 3αHP, 3βHP, or progesterone; the 3αHP-BSA, but not the 3βHP-BSA or the progesterone-BSA, suppressed the GnRH-induced release of FSH. The results of this study provide the first evidence that 3αHP exerts immediate (nongenomic) and direct effects on GnRH-induced FSH release by interacting at the level of the pituitary gonadotroph membrane and the phosphoinositol cell signaling cascade involving Ca2+.
Similar content being viewed by others
References
Clapper, D. L. and Conn, P. M. (1985).Biol. Reprod. 32, 269–278.
Conn, P. M., Huckle, W. R., Andrews, W. V., and McArdle, C. A. (1987).Rec. Prog. Horm. Res. 43, 29–68.
Stojikovic, S. S. and Catt, K. J. (1995).J. Neuroendocrinol. 7, 739–757.
Krey, L. C. and Kamel, F. (1990).Mol. Cell. Endocrinol. 70, 21–29.
Kellom, T. A. and O'Conner, J. L. (1991).J. Steroid Biochem. Molec. Biol. 39, 501–511.
Ravindra, R., and Aronstam, R. S. (1992).Acta Endocrinol. 126, 345–349.
Counis, R. and Jutisz, M. (1991).Trends Endocrinol. Metab. 2, 181–187.
Dhanvantari, S. and Wiebe, J. P. (1994).Endocrinology 134, 371–376.
Wiebe, J. P., Dhanvantari, S., Watson, P. H., and Hang, Y. (1994).Endocrinology 134, 377–382.
Weiss, J. and Jameson, J. L. (1993).TEM 8, 265–269.
Hansen, J. R., McArdle, C. A., and Conn, P. M. (1987).Mol. Endocrinol. 1, 808–815.
McArdle, C. A., and Poch, A. (1992).Endocrinology 130, 3567–3574.
Stojilkovic, S. S., Iida, T., Cesnjaj, M., and Catt, K. J. (1992).Endocrinology 131, 2821–2828.
Liu, T-C. and Jackson, G. L. (1984).Proc. Soc. Exp. Biol. Med. 249, E165-E174.
Kotsuji, F., Winters, S. J., Attardi, B., Keeping, H. S., Oshima, H., and Troen, P. (1988).Endocrinology 123, 2683–2689.
Ortman, O., Wassmann, D., Stojikovic, S. S., Catt, K. J., Schulz, K-D., and Emons, G. (1993).Endocrinology 133, 2632–2638.
Wood, P. H. and Wiebe, J. P. (1989).Endocrinology 134, 377–382.
Wiebe, J. P., de Gannes, G. C., and Dallaire, M. J. (1994).Biol. Reprod. 50, 956–964.
Wiebe, J. P., Boushy, D., and Wolfe, M. (1997).Brain Res. in press.
Li, P-H. S. (1993).Life Sci. 53, 141–151.
Liu, T-C. and Jackson, G. L. (1988).Biol. Reprod. 39, 787–796.
Frawley, L. S. and Neill, J. D. (1984).Endocrinology 114, 659–663.
Kamel, F. and Krey, L. C. (1982).Mol. Cell Endocrinol. 32, 151–164.
Kim, K. and Ramirez, V. D. (1982).Endocrinology 111, 750–757.
Drouin, J. and Jabrie, F. (1981).Endocrinology 108, 52–57.
Morrow, A. L. Pace, J. R., Purdy, R. H., and Paul, S. M. (1990).Molec. Pharmacol. 37, 263–270.
Ortmann, O., Stojilkovic, S. S., Cesnjaj, M., Emons, G., and Catt, K. J. (1992).endocrinology 131, 1565–1567.
Dayanithi, G. and Tapia-Arancibia, L. (1996).J. Neurosci. 16, 130–136.
Ke, F-C. and Ramirez, V. D. (1987).Neuroendocrinology 45, 514–517.
Koch, B., Luz-Bucher, B., Briaud, B., and Mialhe, C. (1987).J. Endocrinol. 79, 215–222.
Bression, D., Michard, M., Le Dafniet, M., Pagesy, P., and Peillon, F. (1986).Endocrinology 119, 1048–1051.
Moore, F. L., Orchinik, M., and Lowry, C. (1995).Receptor 5, 21–28.
Mermelstein, P. G., Becker, J. B., and Surmeier, D. J. (1996).J. Neurosci. 16, 595–604.
Mendoza, C., and Tesarik, J. (1993).FEBS Lett. 330, 57–60.
Wehling, M. (1995).Steroids 60, 153–156.
Wiebe, J. P. and Kavaliers, (1988).Brain Res. 461, 150–157.
Vincens, M., Shu, C., Moguilewsky, M., and Philibert, D. (1989).Eur. J. Pharmacol. 168, 15–21.
Wiebe, J. P. (1997).Rec. Prog. Horm. Res. 52, 71–101.
O'Conner, J. L., Clary, A. R., and Kellom, T. A. (1988).Life Sci. 42, 61–72.
Greeley, G. H., Allen, M. B., and Mahesh, V. B. (1978).Neuroendocrinology 18, 233–241.
Goodman, R. L. (1978).Endocrinology 102, 142–150.
Bourne, G. A., and Baldwin, D. M. (1987).Am. J. Physiol. 253, 290–295.
Drouva, S. V., Gorenne, I., Laplante, E., Rérat, E., Enjalbert, A., and Kordon, C. (1990).Endocrinology 126, 536–544.
Katayama, T. and Conn, P. M. (1994).Endocrinology 134, 119–125.
Conn, P. M., Ganong, B. R., Ebeling, J., Staley, D., Neidel, J., Bell, R. M. (1985).Biochem. Biophys. Res. Commun. 126, 532–538.
Naor, Z., Capponi, A. M., Rossier, M. F., Ayalon, D., and Limor, R. (1988).Mol. Endocrinol. 2, 512–520.
Mendoza, C., Soler, A., and Tesarik, J. (1995).Biochem. Biophys. Res. Commun. 210, 518–523.
Wiebe, J. P., Deline, C., Buckingham, K. D., Dove, V., and Stothers, J. R. (1985).Steroids 45, 39–51.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beck, C.A., Wolfe, M., Murphy, L.D. et al. Acute, nongenomic actions of the neuroactive gonadal steroid, 3α-hydroxy-4-pregnen-20-one (3αHP), on FSH release in perifused rat anterior pituitary cells. Endocr 6, 221–229 (1997). https://doi.org/10.1007/BF02820496
Issue Date:
DOI: https://doi.org/10.1007/BF02820496