Abstract
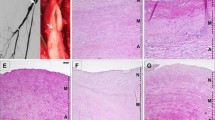
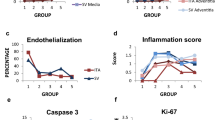
Allotransplantation (TPL) of the abdominal aortic segments of BN donors was performed in 32 Lewis recipients with or without cyclosporin A (CyA) immunosuppression, and the vascular changes were compared to those of 10 syngeneic grafts (Lewis→ Lewis) and to the autologous rat aortae. The vessels were examined 2, 3, 4 and 5 months post TPL by light microscopy, the thickness of intima and media was measured morphometrically and the cell infiltration of adventitia and intima was assessed semiquantitatively. Thirty-six aortae were examined by three-step enzyme immunohistochemistry (proof of selected differentiation, proliferation, cytoskeletal and connective tissue matrix antigens). The adventitia displayed an intense focal and scattered mononuclear cell infiltration: it was more discrete and focal in the intima. This cellularity persisted in the allografts but disappeared from the intima and was reduced in the adventitia of the isografts after four and five months. Disseminated EDI+ activated macrophages were the most prominent population of infiltrates whereas modest numbers of adventitial ED2+ tissue macrophages remained constant throughout the intervals examined CD4+ cells (focal and scattered) outnumbered (roughly twice) the scattered CD8+ lymphocytes; both these types were rare in the intima. Leukocyte invasion of the media was lacking (except for scarce isolated CD8+ cells in some allografts). In syngeneic grafts the smooth muscle cells (SMC) of media remained intact and the intimal thickening was slight to absent (about 5 μm) four and five months post TPL. On the other hand, the allograft media underwent severe destructive changes (karyolysis, depletion of α-SMC actin, focal calcification and general thinning without rupture or aneurysm). The prominent allograft intimal thickening (70–80 μm) was due to the proliferation of longitudinally oriented myointimal cells (α-SMC actin, ED2, PCNA and Ki67+) and an increase in matrix substance (strong metachromasia and positivity of chondroitin-sulfate proteoglycan). The deposition of lipids remained discrete, without atheromatous plaques and mural thrombosis. All changes were comparable in CyA-treated and untreated animals.
Thus the main lesions of the allografts were (i) persistent mononuclear infiltration chiefly in adventitia, (ii) destruction of medial SMC, and (iii) intimal thickening by proliferation of myointimal cells. At the post TPL intervals examined the proliferation and intimal migration of medial SMC were not apparent and a morphological correlate of significant anti-medial-SMC cytotoxic attack was lacking.
Similar content being viewed by others
Abbreviations
- ASMA:
-
α isoform of smooth muscle cell actin
- CHSPG:
-
chondroitin sulfate-proteoglycan
- CyA:
-
cyclosporin A
- DAB:
-
3,3′-diaminobenzidin-tetrahydrochloride
- EA:
-
endarteritis
- HAM:
-
anti-mouse-lg antibody produced in horse
- mAb:
-
monoclonal antibody
- PAS:
-
periodic acid-Schiff staining
- PCNA:
-
proliferating cell nuclear antigen (cyclin)
- PDGF:
-
platelet-derived growth factor
- SMC:
-
smooth muscle cell(s)
- TPL:
-
transplantation
References
Adams D.H., Wyner L.R., Karnovsky M.J.: Experimental graft atherosclerosis.Transplantation 56, 794–799 (1993).
Anderson H.D., Holm P., Sender S. et al.: Relative importance of ischemic injury and immunological injury on the development of transplant atherosclerosis in rabbit aortic allografts.Transplantation 60, 631–638 (1995).
Azuma H., Binder J., Heemann U. et al: Effects of RS 61443 on functional and morphological changes in chronically rejecting rat kidney allografts.Transplantation 59, 460–466 (1995).
Colvin R.B.: The pathogenesis of vascular rejection.Transplant. Proc. 23, 2052–2055 (1991)
Demetris A.J., Zerbe T., Banner B.: Morphology of solid organ allograft arteriopathy.Transplant. Proc. 21, 3667–3670 (1989).
Dennis M.J.S., Beckingham I.J., Blamey R.W.: Evaluation of animal models of chronic vascular rejection.Transplant. Proc. 25, 2102–2103 (1993).
Fellström B., Larsson E., Tufveson G.: Strategies in chronic rejection of transplanted organs—a current view on pathogenesis, diagnosis and treatment.Transplant. Proc. 21, 1435–1439 (1989).
Fellström B.C., Larsson E.: Pathogenesis and treatment perspectives of chronic rejection.Immunol. Rev. 134, 84–98 (1993).
Foegh M.L., Ramwell P.W.: Angiopeptin—experimental and clinical studies of inhibition of myointimal proliferation.Kidney Internat. 48 (Suppl.), 18–22 (1995).
Geerling R.A., De Bruijn R.W.F., Scheringa M. et al.: Suppression of acute rejection prevents graft arteriosclerosis after allogeneic aorta transplantation in the rat.Transplantation 58, 1258–1263 (1994).
Gerlach C., Golding M., Larue L. et al.: Ki-67 immunoexpression is a robust marker of proliferative cells in the rat.Lab. Invest. 77, 697–698 (1997).
Gohra H., McDonald T.O., Vedrrier E.D., Aziz S.: Endothelial loss and regeneration in a model of transplant arteriosclerosis.Transplantation 60, 96–102 (1995).
Gonzalez Z.E., Vermeulen F., Ehrenfeld W.K.: Relations between circulating blood and pathogenesis of atherosclerosis.Israel J. Med. Sci. 5, 648–651 (1969).
Hancock W.W.: Basic science aspects of chronic rejection—induction of protective genes to prevent development of transplant arteriosclerosis.Transplant. Proc. 30, 1585–1589 (1998).
Hansson G.K.: Cytokines regulate proliferation and cytoskeletal organization of vascular smooth muscle cells.Path. Res. Pract. 190, 891–894 (1994).
Häyry P., Isoniemi H., Yilmaz S. et al.: Chronic allograft rejection.Immunol. Rev. 134, 33–81 (1993).
Häyry P.: Pathophysiology of chronic rejection.Transplant. Proc. 28 (Suppl.), 7–10 (1996).
Häyry P., Myllärniemi M., Aavik E. et al.: Role of growth factors in graft vessel disease.Transplant. Proc. 29, 2551–2552 (1997).
Häyry P.: Common pathways in allograft arteriosclerosis and experimental vascular injury—inflammation.Transplant. Proc. 30, 685–686 (1998).
Howie A.J., Bryan R.L., Gunson B.K.: Arteries and veins formed within renal vessels—a previously neglected observation.Virchows Arch. Path. Anat. 420, 301–304 (1992).
Jenkins J., Boyle J.J., McPhaden A.R., Lindop G.B.M.: Three-dimensional reconstruction of abnormal intramural chronic arteries in human heart allograft biopsies.J. Pathol. 181, 247–250 (1997).
Jonasson L., Holm J., Hansson G.K.: Smooth muscle cells express la antigens during arterial response to injury.Lab. Invest. 58, 310–315 (1988).
Koskinen P.K., Lemström K.B., Häyry P.: How cyclosporin modifies histological and molecular events in the vascular wall during chronic rejection of rat cardiac allografts.Amer. J. Pathol. 146, 972–980 (1995).
Lácha J., Lehmann M., Chadimová M. et al.: Effect of anti-CD4 monoclonal antibody and cyclosporin A or a combination of both on chronic rejection in the rat aortic allograft model.Transplant. Proc. 26, 3242–3243 (1994).
Li F., Yin M., van Dam J.G.: Cytomegalovirus infection enhances the neointima formation in rat aortic allografts.Transplantation 65, 1298–1304 (1998).
Maggard M.M., Ke B., Wang T.: Effects of Pravastatin on chronic rejection of rat heart allografts.Transplantation 65, 149–155 (1998).
McDonald P.C., Kenyon J.A., McManus B.M.: The role of lipids in transplant vascular disease.Lab. Invest. 78, 1187–1201 (1998).
McGrath J.A., Eady R.A.J.: Heparan-sulphate proteoglycan and wound healing in skin.J. Pathol. 183, 251–252 (1997).
Mennander A., Tiisala S., Halttunen J. et al.: Chronic rejection in rat aorta allografts—an experimental model for transplant arteriosclerosis.Arterioscl. & Thromb. 11, 671–680 (1991).
Motomura N., Lou H., Orskov H. et al.: Exposure of vascular allografts to IGF-1 increases vascular expression of IGF-1 ligand and receptor protein, and accelerates arteriosclerosis in rats.Transplantation 65, 1024–1030 (1998).
Proudfoot D., Shanahan C.M., Weissberg P.L.: Vascular calcification—new insights into an old problem.J. Pathol. 185, 1–3 (1998).
Rossmann P., Jirka J.:Rejection Nephropathy. Elsevier-North-Holland, Amsterdam, and Academia Publishers, Prague, 1979.
Rossmann P., Jirka J., Chadimová M. et al.: Arteriolosclerosis of the human renal allografts—morphology, origin, life history and relationship to cyclosporin therapy.Virchows Arch. Path. Anat. 418, 129–141 (1991).
Rossmann P., Jirka J., Matoušovic K.:Renal Allograft Biopsy—Image, Interpretation, Interventions. Academic Publishers, Prague 1997.
Rossmann P., Jirka J., Matoušovic K.: Immunohistochemistry of renal allograft biopsies. (In Czech)Čas. Lék. Čes. 137, 757–762 (1998).
Rossmann P., Říhová B., Strohalm J., Ulbrich K.: Morphology of rat kidney and thymus after native and antibody-coupled cyclosporin A application (reduced toxicity of targeted drug).Folia Microbiol. 42, 277–287 (1997).
Russell M.E., Fujita M., Masek M.A.: Cardiac graft vascular disease—nonselective involvement of large and small vessels.Transplantation 56, 1599–1601 (1993).
Ryffel B., Siegel H., Petric R. et al.: Nephrotoxicity of cyclosporin in spontaneously hypertensive rats—effects on blood pressure and vascular lesions.Clin. Nephrol. 25 (Suppl.), 193–198 (1986).
Schmid C., Heemann V., Tilney N.L.: Retransplantation reverses mononuclear infliltration but not myointimal proliferation in a rat model of chronic cardiac allograft rejection.Transplantation 61, 1695–1699 (1996).
Schnitz-Rixen T., Megerman J., Colvin R.B. et al.: Immunosuppressive treatment of aortic allografts.J. Vasc. Surg. 7, 82–92 (1988).
Schwartz R.S.: Neointima and arterial injury: dogs, rats, pigs and more.Lab. Invest. 71, 789–791 (1994).
Solez K., Axelsen R.A., Benediktsson H. et al.: International standardization for the histologic diagnosis of renal allograft rejection—the Banff working classification of kidney transplant pathology.Kidney Internat. 44, 411–422 (1993).
Subramanian S.V., Orosz C.G., Stranch A.R.: Vascular smooth muscle cell alpha actin as an indicator of parenchymal cell reprogramming in heart allografts.Transplantation 65, 1652–1656 (1998).
Tanabe S., Ueda M., Han Y.S. et al.: Enhanced fibronectin expression is associated with the development of graft arteriosclerosis in human renal allografts.Transplant. Proc. 27, 1078–1081 (1995).
Tilney N.L., Whitley W.D.W., Diamond J.R. et al.: Chronic rejection—an underfined conundrum.Transplantation 52, 389–398 (1991).
Vanrenterghem Y.: The use of mycophenolate—Mofetil in renal transplantation.Nephron 76, 392–399 (1997).
Wilhelm M.J., Kusaka M., Pratschke J., Tilney N.L.: Chronic rejection—increasing evidence for the importance of allogen-independent factors.Transplant. Proc. 30, 2402–2406 (1998).
Xiao F., Chong A., Shen J. et al.: Pharmacologically induced regression of chronic transplant rejection.Transplantation 60, 1065–1072 (1995).
Yilmaz S., Häyry P.: The impact of acute episodes of rejection on the generation of chronic rejection in rat renal allografts.Transplantation 56, 1153–1156 (1993).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rossmann, P., Lácha, L. & Lodererová, A. Morphology and immunohistochemistry of rat aortic grafts. Folia Microbiol 44, 339–353 (1999). https://doi.org/10.1007/BF02818558
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02818558