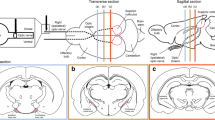
Abstract
Activated astrocytes, intrinsic components of both local and remote (axonal target regions) central nervous system injury responses, are now recognized as active metabolic and regulatory mediators in many neurological disorders. To further define these responses, we devised a new ventral surgical approach to unilaterally lesion the inferior olivary nuclear complex, which has a single predominant remote target, the cerebellum. Activated astrocyte number, volume, and density, as well as the total volume of brainstem involved in the astrocytic response, all peaked at postlesion day (pld) 4, returning toward, but not to, unoperated control values at pld 24 (p<0.05). In contrast, the peak astrocyte response in the cerebellum was delayed, being greatest at pld 6 (p<0.05 compared to control or pld 2). These responses were associated with increases in overexpression of S100β, an astrocyte-derived neurite growth factor, and with an increase in cerebellar steady-state levels of a neuronal injury response protein, the β-amyloid precursor protein (β-APP). This is similar to correlated increases in these two proteins that are found in epilepsy and alzheimer disease. Our studies defining remote astrocytic and neuronal responses may be important for understanding glialneuronal mechanisms underlying the spread of neuropathological changes in conditions such as Alzheimer disease.
Similar content being viewed by others
References
Azizi S. A. and Woodward D. J. (1987) Inferior olivary nuclear complex of the rat: morphology and comments on the principles of organization within the olivocerebellar system.J. Comp. Neurol. 263, 467–484.
Bardin J. M., Batini C., Billard J. M., Buisseret-Delmas C., Conrath-Verrier M., and Corvaja N. (1983) Cerebellar output regulation by the climbing and mossy fibers with and without the inferior olive.J. Comp. Neurol. 213, 464–477.
Batini C., Daniel H., and Ramirez R. D. (1987) Release of cerebellar inhibitory activity by partial destruction of the inferior olive with kainic acid in rat.Brain Res. 403, 186–191.
Benedetti F., Montarolo P. G., and Rabacchi, S. (1984) Inferior olive lesion induces longlasting functional modification in the Purkinje cells.Exp. Brain Res. 55, 368–371.
Braak H. and Braak E. (1995) Staging of Alzheimer's disease-related neurofibrillary changes.Neurobiol. Aging 16, 271–278.
Braak H. and Braak E. (1991) Neuropathological stageing of Alzheimer-related changes.Acta Neuropathol. (Berl) 82, 239–259.
Cavanaugh J. B. (1970) The proliferation of astrocytes around a needle wound in the rat brain.J. Anat. 106, 471–487.
Chan-Palay V., Palay S. L., Brown J. T., and Van Itallie C. (1977) Sagittal organization of olivocerebellar and reticulocerebellar projections: Autoradiographic studies with35S-methionine.Exp. Brain Res. 30, 561–576.
Condorelli D. F., Dell'Albani P., Kaczmarek L., Messina L., Spampinato G., Avola R., Messina A., and Giuffrida Stella A. M. (1990) Glial fibrillary acidic protein messenger RNA and glutamine synthetase activity after nervous system injury.J. Neurosci. Res. 26, 251–257.
da Cunha A., Jefferson J. J., Tyor W. R., Glass J. D., Jannotta F. S., and Vitkovic L. (1993) Gliosis in human brain: relationship to size but not other properies of astrocytes.Brain Res. 600, 161–165.
Eng L. F., Vanderhaeghen J. J., Bignami A., and Gerstl B. (1971) An acidic protein isolated from fibrous astrocytes.Brain Res. 28, 351–354.
Fedoroff S., Ahmed I., and Wang E. (1990) The relationship of expression of statin, the nuclear protein of nonproliferating cells, to the differentiation and cell cycle of astroglia in cultures andin situ.J. Neurosci. Res. 26, 1–15.
Forloni G., Demicheli F., Giorgi S., Bendotti C., and Angeretti N. (1992) Expression of amyloid precursor protein mRNAs in endothelial, neuronal and glial cells: modulation by interleukin-1.Brain Res. Molec. Brain Res. 16, 128–134.
Gilmore S. A., Sims T. J., and Leiting J. E. (1990) Astrocytic reactions in spinal gray matter following sciatic axotomy.Glia 3, 342–349.
Giulian D. and Lachman L. B. (1985) Interleukin-1 stimulation of astroglial proliferation after brain injury.Science 228, 497, 498.
Griffin W. S. T. (1987) Methods for hybridization and quantitation of mRNA in individual brain cells, inIn Situ Hybridization: Applications to Neurobiology (Barchas J. D., ed.), pp. 97–110, Oxford University Press, New York.
Griffin W. S. T., Sheng J. G., Gentleman S. M., Graham D. I., Mrak R. E., and Roberts G. W. (1994) Microglial interleukin-1α expression in human head injury: correlations with neuronial and neuritic β-amyloid precursor protein expression.Neurosci. Lett. 176, 133–136.
Griffin W. S. T., Stanley L. C., Ling C., White L., MacLeod V., Perrot L. J., White III C. L., and Araoz C. (1989) Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease.Proc. Natl. Acad. Sci. USA 86, 7611–7615.
Griffin W. S. T., Stanley L. C., Yeralan O., Rovnaghi C. R., and Marshak D. R. (1993) Methods for the study of cytokines in human neurodegenerative disease, inMethods in Neuroscience, vol. 17 (De Souza E. B., ed.), pp. 268–287, Academic, Orlando, FL.
Griffin W. S. T., Yeralan O., Boop F., Rovnaghi C. R., Sheng J. G., Mrak R. E. and Van Eldik L. C. (1995) Overexpression of the neurotropic cytokine S100β in human temporal lobe epilepsy.J. Neurochem. 65, 228–233.
Guntinas-Lichius O., Neiss W. F., Gunkel A., and Stennert E. (1994) Differences in glial, synaptic and motoneuron responses in the facial nucleus of the rat brainstem following facial nerve resection and nerve suture reanastomosis.Eur. Arch. Otorhinolaryngol. 251, 410–417.
Hetier E., Ayala J., Denefle P., Bousseau A., Rouget P., Mallat M., and Prochiantz A. (1988) Brain macrophages synthesize interleukin-1 and interleukin-1 mRNAsin vitro.J. Neurosci. Res. 21, 391–397.
Hozumi I., Aquino D. A., and Norton W. T. (1990a) GFAP mRNA levels following stab wounds in rat brain.Brain Res. 534, 291–294.
Hozumi I., Chiu F. C., and Norton W. T. (1990b) Biochemical and immunocytochemical changes in glial fibrillary acidic protein after stab wounds.Brain Res. 524, 64–71.
Jensen M. B., Gonzalez B., Castellano B., and Zimmer J. (1994) Microglial and astroglial reactions to anterograde axonal degeneration: a histochemical and immunocytochemical study of the adult rat fascia dentata after entorhinal perforant path lesions.Exp. Brain Res. 98, 245–260.
Kiefer R., Lindholm D., and Kreutzberg G. W. (1993) Interleukin-6 and transforming growth factor-beta 1 mRNAs are induced in rat facial nucleus following motoneuron axotomy.Eur. J. Neurosci. 5, 775–781.
Latov N., Nilaver G., Zimmerman E. A., Johnson W. G., Silverman A. J., Defendini R., and Cote L. (1979) Fibrillary astrocytes proliferate in response to brain injury, A study combining immunoperoxidase technique for glial fibrillary acidic protein and radioautography of tritiated thymidine.Dev. Biol. 72, 381–384.
Marshak D. R., Pesce S. A., Stanley L. C., and Griffin W. S. T. (1991) Increased S100β neurotrophic activity in Alzheimer disease temporal lobe.Neurobiol. Aging 13, 1–7.
Mathewson A. J. and Berry M. (1985) Observations on the astrocyte response to a cerebral stab wound in adult rats.Brain Res. 327, 6–69.
Miller R. H., Abney E. R., David S., Ffrench-Constant C., Lindsay R., Patel R., Stone J., and Raff M. C. (1986) Is reactive gliosis a property of a distinct subpopulation of astrocytes?J. Neurosci. 6, 22–29.
Miyake T., Hattori T., Fukuda M., Kitamura T., and Fujita S. (1988) Quantitative studies on proliferative changes of reactive astrocytes in mouse cerebral cortex.Brain Res. 451, 133–138.
Montarolo P. G., Palestini M., and Strata P. (1982) The inhibitory effect of the olivocerebellar input on the cerebellar purkinje cells in the rat.J. Physiol. 332, 187–202.
Mrak R. E., Sheng J. G., and Griffin W. S. T. (1995) Glial cytokines in Alzheimer's disease: Review and pathogenic implications.Human Pathol. 26, 816–823.
Mrak R. E., Sheng J. G., and Griffin W. S. T. (1996) Correlation of astrocytic S100β expression with dystrophic neurites in amyloid plaques of Alzheimer's disease.J. Neuropathol. Exp. Neurol. 55, 273–279.
Oltmans G. A., Lorden J., and Beales M. (1985) Lesions of the inferior olive increase glutamic acid decarboxylase activity in the deep cerebellar nuclei of the rat.Brain Res. 347, 154–158.
Paxinos G. and Watson C. (1976)The Rat Brain in Stereotaxic Coordinates, 2nd ed Academic, Sydney, Australia.
Poirier J., Hess M., May P. C., and Finch C. E. (1991a) Astrocytic apolipoprotein E mRNA and GFAP mRNA in hippocampus after entorhinal cortex lesioning.Brain Res. 11, 97–106.
Poirier J., Hess, M., May P. C., and Finch C. E. (1991b) Cloning of hippocampal poly(A) RNA sequences that increase after entorhinal cortex lesion in adult rat.Molec. Brain Res. 9, 191–195.
Rio-Hortega P. D. and Penfield W. (1927) Cerebral cicatrix.Bull. Johns Hopkins Hosp. 41, 278–303.
Schiffer D., Giordana M. T., Migheli A., Giaccone G., Pezzotta S., and Mauro A. (1987) Glial fibrillary acidic protein and vimentin in the experimental glial reaction of the rat brain.Brain Res. 374, 110–118.
Sheng J. G., Mrak R. E., and Griffin W. S. T. (1994) S100β protein expression in Alzheimer's disease: potential role in the pathogenesis of neuritic plaques.J. Neurosci. Res. 39, 398–404.
Sheng J. G., Mrak R. E., Rovnaghi C. R., Kozlowska E., Van Eldik L. J., and Griffin W. S. T. (1996a) Human brain S100β and S100β mRNA expression increases with age: pathogenic implications for Alzheimer's disease.Neurobiol. Aging 17, 359–363.
Sheng J. G., Ito K., Skinner R. D., Mrak R. E., Rovnaghi C. R., Van Eldik L. J., and Griffin W. S. T. (1996b)In vivo andin vitro evidence supporting a role for the inflammatory cytokine interleukin-1 as a driving force in Alzheimer pathogenesis.Neurobiol. Aging 17, 761–766.
Shinoda H., Marini A. M., Cosi C., and Schwartz J. P. (1989) Brain region and gene specificity of neuropeptide gene expression in cultured astrocytes.Science 245, 415–417.
Sola C., Garcia-Ladona F. J., Sarasa M., Mengod G., Probst A., and Palacios J. M. (1993) β-APP gene expression is increased in the rat brain after motor neuron axotomy.J. Neurosci. 5, 795–808.
Stanley L. C., Mrak R. E., Woody R. C., Perrot L. J., Zhang S.-X., Marshak D. R., Nelson S. J., and Griffin W. S. T. (1994) Glial cytokines as neuropathogenic factors in HIV infection: Pathogenic similarities to Alzheimer's disease.J. Neuropathol. Exp. Neurol. 53, 231–238.
Steward O., Torre E. R., Phillips L. L., and Trimmer P. A. (1990) The process of reinnervation in the dentate gyrus of adult rats: time course of increases in mRNA for glial fibrillary acidic protein.J. Neurosci. 10, 2373–2384.
Streit W. J. and Kreutzberg G. W. (1988) Response of endogenous glial cells to motor neuron degeneration induced by toxic ricin.J. Comp. Neurol. 268, 248–263.
Takamiya Y., Kohsaka S., Toya S., Otani M., and Tsukada Y. (1988) Immunohistochemical studies on the proliferation of reactive astrocytes and the expression of cytoskeletal proteins following brain injury in rats.Dev. Brain Res. 38, 201–210.
Van Eldik L. J. and Griffin W. S. T. (1994) S100β expression in Alzheimer's disease: Relation to neuropathology in brain regions.Biochim. Biophys. Acta 39, 398–404.
Vaughn J. E., Hinds P. L., and Skoff R. P. (1970) Electron microscopic studies of Wallerian degeneration in rat optic nerves. I. The multipotential glia.J. Comp. Neurol. 140, 175–206.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ito, K., Ishikawa, Y., Skinner, R.D. et al. Lesioning of the inferior olive using a ventral surgical approach. Molecular and Chemical Neuropathology 31, 245–264 (1997). https://doi.org/10.1007/BF02815128
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02815128