Abstract
Tissue digestion prior to analysis for trace metals is usually carried out with strong acids. Nitric acid, alone or in combination with perchloric acid, is most commonly used. In addition to the laborious acid washing of all glassware prior to use, the digestion necessitates exposure to potential environmental contamination. Use of perchloric acid mandates a specially constructed hood with facilities for washing to remove acid deposits.
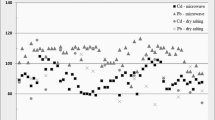
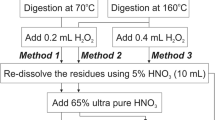
A simple digestion procedure using 30% hydrogen peroxide in polyethylene vials in an oven at approximately 75°C has been previously described for the measurement of zinc in tissues using flame or flameless atomic absorption spectrophotometry and of selenium in liver by flameless atomic absorption. Readings for reagent blanks were insignificant. The technique has been further developed with a reduction in digestion time using 50% H2O2. Analysis of liver has been extended to include copper, manganese, and arsenic. Although the level of arsenic present was too low to be detected, 50 and 100 ng of this element added to the liver powder was completely recovered.
The digest obtained when dissolved in appropriate solvent is suitable for analysis for multiple trace metals.
Similar content being viewed by others
References
M. S. Clegg, C. L. Keen, B. Onnerdal, and L. S. Hurley,Biol. Trace Elem. Res. 3, 107 (1981).
M. S. Clegg, C. L. Keen, B. Lonnerdal, and L. S. Hurley,Biol. Trace Elem. Res. 3, 237 (1981).
J. Versieck, J. Hoste, F. Barbier, H. Steyaert, J. De Rudder, H. Michels,Clin. Chem. 24, 303 (1978).
J. Smeyers-Verbeke, E. Defrise-Gussenhoven, G. Ebinger, A. Lowenthal, and D. L. Massart,Clin. Chim. Acta 51, 309 (1974).
A. C. Greiner, S. C. Chan, and G. A. Nicolson,Clin. Chim. Acta 64, 211 (1975).
J. J. Valdes, S. W. Hartwell, S. Sato, and J. M. Frazier,Pharmacol. Biochem. Behav. 16, 915 (1982).
M. A. Klitenick, C. J. Frederickson, and W. I. Manton,Anal. Chem. 55, 921 (1983).
C. Chang and S. Bloom,J. Am. Coll. Nutr. 2, 149 (1983).
N. W. Alcock, inthe Neurobiology of Zinc, Part A:Physiochemistry, Anatomy and Techniques, Alan R. Liss, New York, NY, 1984, pp. 305–316.
N. W. Alcock,Proceedings of the 3rd International Symposium on Selenium, Beijing, 1983 (In Press).
N. W. Alcock, inAm. Chem. Soc. Abstr. (Analytical Section), Abstract No. 50 (1985).
Author information
Authors and Affiliations
Additional information
Author to whom all correspondence and reprint requests should be addressed.
Rights and permissions
About this article
Cite this article
Alcock, N.W. A hydrogen-peroxide digestion system for tissue trace-metal analysis. Biol Trace Elem Res 13, 363–370 (1987). https://doi.org/10.1007/BF02796647
Issue Date:
DOI: https://doi.org/10.1007/BF02796647