Summary
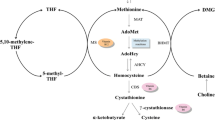
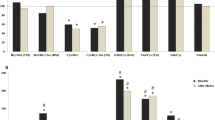
Serum methionine levels increased to a greater extent in patients with severe liver diseases such as fulminant hepatitis and liver cirrhosis with and without hepatic encephalopathy. However, the concentrations remained unchanged in non-encephalopathic cirrhotic cases associated with hepatocellular carcinoma, and their serum methionine levels increased only moderately even at the time of encephalopathy. At least two different mechanisms of serum methionine elevations, possibly due to release from injured hepatocytes or diminished catabolisms of this amino acid in the damaged liver, could be differentiated; the former would be involved mainly in fulminant hepatitis and the latter in liver cirrhosis. A methionine-loading test performed in cirrhotic patients supported the validity of these considerations. No significant increase of serum methionine levels in cirrhotic patients with hepatocellular carcinoma was observed, possibly by remarkable consumption of this amino acid in hepatoma tissues. During the clinical course of several patients, serial determinations of serum methionine concentrations indicated that the levels varied depending upon alterations in the pathophysiological state of the damaged liver; much higher levels were observed concomitantly with decompensated signs such as ascites, jaundice and hepatic encephalopathy. These results suggest that monitoring of serum methionine levels would be very valuable, especially for judging prognosis and predicting hepatic encephalopathy in severe liver disease.
Similar content being viewed by others
References
Fischer JE, et al: Plasma amino acid in patient with hepatic encephalopathy -Effect of amino acid solution. Am J Surg 127: 40, 1974
Record CO, et al: Plasma and brain amino acids in fulminant hepatic failure and their relationship to hepatic encephalopathy. Europ J Clin Invest 6: 387, 1976
Watanabe A, et al: Serum amino acids in hepatic encephalopathy -Effect of branched-chain amino acid infusion of serum aminogram. Acta Hepato-Gastroenterol 26:36, 1979
Shults WT, et al: Methanethiol poisoning. JAMA 211: 2153, 1970
Zieve L, et al: Synergism between marcaptans and ammonia or fatty acids in the production of coma: A possible role for mercaptans in pathogenesis of hepatic coma. J Lab Clin Med 83: 16, 1974
Merino GE, et al: Methionine-induced hepatic coma in dogs. Am J Surg 130: 41, 1975
Kosaka K: Symposium (I) Fulminant hepatitis. Jpn J Gastroenterol 68: 855, 1971
Sherlock S: Hepato-cellular failure, in “Diseases of the liver and biliary system” Vol 5, Asian edition, by Sherlock S, Tokyo, 1975, p 87
Watanabe A, et al: An approach to nutritional therapy of hepatic encephalopathy by normalization of deranged amino acid patterns in serum. Acta Med Okayama 32: 427, 1978
Watanabe A, et al: A rapid method for monitoring serum neutral amino acid concentrations in severe liver diseases. Hepato-Gastroenterol 28: 17, 1981
Higashi T, et al: Impaired metabolism of methionine in patients with severe liver disease (3) -Clinical significance of an intravenous methionine-loading test. Jpn J Gastroenterol 76: 546, 1979
Kunin CM, et al: Persistence of antibiotics in blood of patients with acute renal failure. II. Chloramphenicol and its metabolic products in the blood of patients with severe renal disease or hepatic cirrhosis. J Clin Invest 38: 1498, 1959
Higashi T, et al: Clinical experimental studies on characteristic features of serum aminogram in cirrhotic patients with primary hepatoma and in rats with ethionine-induced hepatoma. Acta Hep Jpn 21: 1196, 1980
Felig P, et al: Amino acid metabolism during prolonged starvation. J Clin Invest 48: 584, 1969
Brodam V, et al: The effect of glucagon on free plasma amino acids in cirrhotics and healthy controls. Acta Hepato-Gastroenterol 25: 23, 1978
Goldberg AL, et al: Regulation and significance of amino acid metabolism in skeletal muscle. Fed Proc 37: 2301, 1978
Sherwin R, et al: Hyperglucagonemia in Laennec’s cirrhosis. The role of portal-systemic shunting. New Engl J Med 290: 239, 1974
Watanabe A, et al: Clinical significance of branchedchain amino acid infusion during treatment of patients with fulminant hepatitis. Acta Hep Jpn 20: 702, 1979
Aguirre A, et al: Plasma amino acids in dogs with two experimental forms of liver damage. J Surg Res 16: 339, 1974
Rosen HM, et al: Plasma amino acid patterns in hepatic encephalopathy of differing etiology. Gastroenterology 72: 483, 1977
Cantoni GL: S-Adenosylmethionine: Present status and future perspectives. The biochemistry of adenosylmethionine by Salvatore F, Borek E, Zappia V, Williams-Ashman HG, Schlenk F. Columbia University Press, New York, 1977, p 558
Gaull GE, et al: Methionine-adenosyl transferase deficiency: New enzymatic defect associated with hypermethionemia: Science 186: 59, 1974
Gaull GE, et al: Biochemical observation so-called hereditary tyrosinemia. Pediat Res 4: 337, 1970
Smuckler EA, et al: Cellular adenosine triphosphate levels in liver and kidney during CCl4 intoxication. Lab Invest 19: 218, 1968
Labadarious D, et al: Vitamin B6 deficiency in chronic liver disease -Evidence for increased degradation of pyridoxal-5’-phosphate. Gut 18: 23, 1977
Goseki K, et al: Combined therapy of anticancer agents and total parenteral nutrition using methionine-deficiency amino acid solution for gastrointestinal tumor. Jpn J Gastroenterol 78: 629, 1981
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Higashi, T. Impaired metabolism of methionine in severe liver diseases I. Clinical and pathophysiological significance of elevated serum methionine levels. Gastroenterol Jpn 17, 117–124 (1982). https://doi.org/10.1007/BF02774550
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02774550