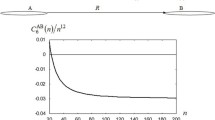
Abstract
The interatomic potentials in a system formed by an ion of an inert gas in the ground state and an atom of an inert gas (e.g., Ne+, Ar+-Ne, Ar, Kr, Xe) are calculated on the basis of a calculation of the most important polarization diagrams of perturbation theory in the Thomas-Fermi approximation. The calculation employs the effective pseudopotential method using a new form of the polarization interaction potential. Results are presented from a calculation of the quasimolecular terms of particular van der Waals systems that improve existing data; some of the data were obtained in earlier studies.
Similar content being viewed by others
References
A. A. Radtsig and B. M. Smirnov, Handbook on Atomic and Molecular Physics [in Russian], Energoatomizdat, Moscow (1986).
E. E. Nikitin and S. Ya. Umanskii, "Semiempirical methods of calculating the interaction potentials of atoms," Itogi Nauki Tekh. Ser. Stroenie Mol. Khim. Syaz’ (VINITI), No. 4 (1980).
H. Haberland, Y. T. Lee, and P. E. Siska, in: Excited States in Chemical Physics, Vol. 2, J. W. McGowan (ed.): Advances in Chemical Physics, Wiley, New York (1981), Vol. 45, Chap. 6, pp. 457–478.
P. Durand and J. P. Malriew, in:Ab initio Methods of Quantum Chemistry, K. P. Lawley (ed.), Wiley, New York (1987).
U. Buck, Rev. Mod. Phys.,46, 369–394 (1974).
A. Z. Devdariani and A. L. Zagrebin, in: Chemistry of Plasma [in Russian], No. 15, B. M. Smirnov (ed.), Moscow (1989), pp. 44–93.
A. L. Zagrebin and S. I. Tserkovnyi, Khim. Fiz.,10, 595–606 (1991).
W. E. Baylis, J. Chem. Phys.,51, 2665–2674 (1969).
D. Duren, Chem. Phys. Lett.,39, No. 3, 481–485 (1976).
A. V. Glushkov, Zh. Strukt. Khim.,36, No. 4, 608–614 (1995).
A. V. Glushkov, Opt. Spektrosk.,77, No. 1, 5–10 (1994).
A. V. Glushkov, Opt. Spektrosk.,77, No. 6, 41–48 (1994).
C. Bottcher and A. Dalgarno, Proc. Roy. Soc. London A,340, 187–196 (1974).
G. K. Ivanov, Teor. Éksp. Khim.,14, No. 1, 110–115 (1978);15, No. 1, 44–50 (1979).
A. V. Glushkov, Zh. Fiz. Khim.,66, No. 6, 1259–1268 (1992).
I. G. Kaplan, Introduction to the Theory of Intermolecular Interactions [in Russian], Nauka, Moscow (1982).
P. Gombas, The Pseudopotential [in German], Springer-Verlag (1967).
A. V. Glushkov, S. V. Ambrosov, T. V. Buyadzhi, et al., Zh. Prikl. Spektrosk.,64, No. 2, 260–263 (1996).
E. P. Ivanova, L. N. Ivanov, A. V. Glushkov, and A. E. Kramida, Phys. Scripta,32, No. 4, 512–524 (1985).
A. V. Glushkov and E. P. Ivanova, J. Quant. Spectr. Rad. Transf.,36, No. 2, 127–145 (1986).
A. V. Glushkov, Zh. Strukt. Khim.,29, No. 4, 3–10 (1988);31, No. 1, 13–18 (1990).
A. V. Glushkov, Zh. Fiz. Khim.,63, No. 11, 2895–2898 (1989);66, No. 4, 589–595 (1992).
V. V. Tolmachev, Adv. Chem. Phys.,14, 421–482 (1969).
V. V. Tolmachev, L. N. Ivanov, and E. P. Ivanova, Izv. Vyssh. Uchebn. Zaved., Fiz., No. 12, 84–89 (1969).
V. S. Letochov and L. N. Ivanov, Comm. At. Mol. Phys. D., No. 4, 169–192 (1985).
A. V. Glushkov, S. V. Ambrosov, V. E. Orlova, and S. V. Orlov, Proc. 5th Atomic Spectra and Oscillator Strengths for Astrophysical and Laboratory Plasma Conf. (5th ASOSALP), Meudon, Paris (1995).
Additional information
Odessa Hydrometeorological Institute. Translated from Izvestiya Vysshikh Uchebnykh Zavedenii, Fizika, No. 3, pp. 36–40, March, 1998.
Rights and permissions
About this article
Cite this article
Glushkov, A.V., Kivganov, A.F., Khokhlov, V.N. et al. Calculation of the spectroscopic characteristics of biatomic van der Waals molecules and ions: Inert gas atom — halogen-type inert gas ion in the ground state. Russ Phys J 41, 223–226 (1998). https://doi.org/10.1007/BF02766415
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02766415