Abstract
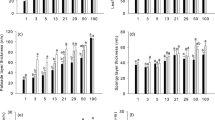
Photosynthetic responses to a series of 1-min lightflecks (1,000µmol m−2 s−1) superimposed on a background with different duration (1, 5, and 10 min) and intensity (25 and 50µmol m−2 s−1) of low background photosynthetic photon flux density (PPFD) were measured in the leaves ofFagus crenata grown in a gap and understory of aFagus crenata forest in the Naeba Mountains. The two background PPFD intensities most frequently occurred in understory and gap sites respectively. The maximum net photosynthetic rate (P Nmax) and maximum stomatal conductance (g smax) were higher in the gap seedlings than in the understory seedlings. However, when the background PPFD was 25µmol m−2s−1, the net photosynthetic rate (P 25) and stomatal conductance (g s25) were almost the same between the gap and understory. When the background PPFD duration was 1-min, the net photosynthetic rate (P N ) at the end of each lightfleck increased progressively. When the background PPFD duration was 5- and 10-min, the increase inP N at the end of each lightfleck was less. This indicates that background PPFD duration is important to photosynthetic responses to lightflecks. The higher ratios ofP 25/P Nmax andg s25/g smax in the understory seedlings indicate that the understory seedlings can maintain relatively lower levels of biochemical and stomatal limitations than the gap seedlings under low light conditions. The ratios ofP N /P Nmax at the end of each lightfleck (IS) and light utilization efficiency of single lightflecks (LUE s) that showed the influence of lightflecks on carbon gain were higher in the understory seedlings than in the gap seedlings when the background PPFD was 25µmol m−2 s−1. This means that understory seedling are capable of utilizing fluctuating light more efficiently under low light conditions than the gap seedlings although the net carbon gain of single lightflecks (CG s) in the understory seedlings was not higher than that in the gap seedlings. There were no significant differences inIS andLUE s between understory seedlings at a background PPFD of 25µmol m−2 s−1 and gap seedlings at a background PPFD of 50µmol m−2 s−1. However,CG s in gap seedlings was higher than in understory seedlings. These results provide more evidence thatF. crenata acclimate to a natural light environment in respect to relative induction state at low background PPFD and can capture the fluctuating light at the same efficiency in both the gap and understory seedlings under natural light environments.
Similar content being viewed by others
Literature cited
Boardman, N.K. (1977) Comparative photosynthesis of sun and shade plants. Ann. Rev. Plant Physiol. 28: 355–377.
Chazdon, R.L. (1986) Light variation and carbon gain in rain forest understory palms. J. Ecol. 74: 995–1012.
Chazdon, R.L. (1988) Sunflecks and their importance to forest understory plants. Adv. Ecol. Res. 18: 1–63.
Chazdon, R.L. and Pearcy, R.W. (1986a) Photosynthetic responses to light variation in rain forest species. I. Induction under constant and fluctuating conditions. Oecologia 69: 517–523.
Chazdon, R.L. and Pearcy, R.W. (1986b) Photosynthetic responses to light variation in rain forest species. II. Carbon gain and photosynthetic efficiency during lightflecks. Oecologia 69: 524–531.
Chazdon, R.L. and Pearcy, R.W. (1991) The importance of sunflecks for forest understory plants. Bioscience 41: 760–766.
Chen, H.Y.H. and Klinka, K. (1997) Light availability and photosynthesis ofPseudotsuga menziesii seedlings grown in the open and the forest understorey. Tree Physiol. 17: 23–29.
Crews, C.E., Vines, H.M., and Black, C.C.Jr. (1975) Postillumination burst of carbon dioxide in crassulacean acid metabolism plants. Plant Physiol. 55: 652–657.
Decker, J.P. (1959) Comparative responses of carbon gain dioxide outburst and uptake in tabacco. Plant Physiol. 34: 100–102.
Han, Q., Yamaguchi, E., Odaka, N., and Kakubari, Y. (1999) Photosynthetic induction responses to variable light under field conditions in three species grown in the gap and understory of aFagus crenata forest. Tree Physiol. 19: 625–634.
Ishizuka, K. (1974) Mountain vegetation.In The flora and vegetation of Japan. Numata, M. (ed.), 294pp, Kodansha, Tokyo, 173–210.
Kirschbaum, M.U.F. and Pearcy, R.W. (1988) Gas exchange analysis of relative importance stomatal and biochemical factors in photosynthetic induction inAlocasia macrorrhiza. Plant Physiol. 86: 782–785.
Kitajima, K. (1994) Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia 98: 419–428.
Knapp, A.K. (1992) Leaf gas exchange inQuercus macrocarpa (Fagaceae): Rapid stomatal responses to variability in sunlight in a tree growth form. Am. J. Bot. 79: 599–604.
Knapp, A.K., Smith, W.K., and Young, D.R. (1989) Importance of intermittent shade to the ecophysiology of subalpine herbs. Funct. Ecol. 3: 753–758.
Koizumi, H. and Oshima, Y. (1993) Light environment and carbon gain of understory herbs associated with sunflecks in a warm temperate deciduous forest. Ecol. Res. 8: 135–142.
Kursar, T.A. and Coley, P.D. (1993) Photosynthetic induction times in shade tolerant species with long and short-lived leaves. Oecologia 93: 165–170.
Liang, N., Nagayama, M., Nakata, M., and Maruyama, K. (1995) Growth, photosynthesis and nitrogen content in Japanese beech (Fagus crenata Bl.) seedlings grown under five irradiances. Photosynthetica 31: 257–268.
Murchie, E.H. and Horton, P. (1997) Acclimation of photosynthesis to irradiance and spectral quality in British plant species: Cholorphyll content, photosynthetic capacity and habitat preference. Plant Cell Environ. 20: 438–448.
Naramoto, M., Han, Q., and Kakubari, Y. (2001) The influence of previous irradiance on photosynthetic induction in three species grown in the gap and understory of aFagus crenata forest. Photosynthetica 39: 545–552.
Pearcy, R.W. (1987) Photosynthetic gas exchange responses of Australian tropical forest trees in canopy, gap and understory microenvironments. Funct. Ecol. 1: 169–178.
Pearcy, R.W. (1990) Sunflecks and photosynthesis in plant canopies. Annu. Rev. Plant Physiol. Plant Mol. Biol. 41: 421–453.
Pearcy, R.W. and Calkin, H.W. (1983) Carbon dioxide exchange of C3 and C4 tree species in the understory of Hawaiian forest. Oecologia 58: 26–32.
Pearcy, R.W., Krall, J.P., and Sassenrath-Cole, G.F. (1996) Photosynthesis in fluctuating light environments.In Photosynthesis and the environment. Baker, N.R. (ed.), 491pp, Kluwer Academic Publishers, 321–346.
Pfitsch, W.A. and Pearcy, R.W. (1989) Daily carbon gain byAdenocaulon bicolor (Asteraceae), a redwood forest understory herb, in relation to its light environment. Oecologia 80: 465–470.
Pons, T.L. and Pearcy, R.W. (1992) Photosynthesis in flashing light in soybean leaves grown in different conditions. II. Lightfleck utilization efficiency. Plant Cell Environ. 15: 577–584.
Pons, T.L., Pearcy, R.W., and Seemann, J.R. (1992) Photosynthesis in flashing light in soybean leaves grown in different conditions. I. Photosynthetic induction state and regulation of ribulose-1,5-bisphosphate carboxylase activity. Plant Cell Environ. 15: 569–576.
Poorter, L. and Oberbauer, S.F. (1993) Photosynthetic induction response of two rainforest tree species in relation to light environment. Oecologia 96: 193–199.
Sassenrath-Cole, G.F. and Pearcy, R.W. (1992) The role of ribulose-1,5-bisphosphate regeneration in the induction requirement of photosynthetic CO2 exchange under transient light conditions. Plant Physiol. 99: 227–234.
Sassenrath-Cole, G.F. and Pearcy, R.W. (1994) Regulation of photosynthetic induction state by magnitude and duration of low light exposure. Plant Physiol. 105: 1115–1123.
Seemann, J.R., Kirschbaum, M.U.F., Sharkey, T.D., and Pearcy, R.W. (1988) Regulation of ribulose-1,5-bisphosphoate carboxylase activity inAlocasia macrorrhiza in response to step changes in irradiance. Plant Physiol. 88: 148–152.
Tang, Y., Koizumi, H., Satoh, M., and Washitani, I. (1994) Characteristics of transient photosynthesis inQuercus serrata seedlings grown under lightfleck and constant regimes. Oecologia 100: 463–369.
Tinoco-Ojanguren, C. and Pearcy, R.W. (1992) Dynamic stomatal behavior and its role in carbon gain during sunflecks of a gap phase and an understory Piper species acclimated to high and low light. Oecologia 92: 222–228.
Valladares, F., Allen, M.T., and Pearcy, R.W. (1997) Photosynthetic response to dynamic light under field conditions in six tropical rainforest shrubs occuring along a light gradient. Oecologia 111: 505–514.
Watanabe, S. (1994) Specia of trees. 450pp, University of Tokyo Press, Tokyo. (in Japanese)
Weber, J.A., Jurik, T.W., Tenhunen, J.D., and Gates, D.M. (1985) Analysis of gas exchange in seedlings ofAcer saccharum: Integration of field and laboratory studies. Oecologia 65: 338–347.
Author information
Authors and Affiliations
Additional information
This study was funded by the research project, Evaluation of Total CO2 Budget in Forest Ecosystems, coordinated by the Ministry of Agriculture, Forestry and Fisheries of Japan.
About this article
Cite this article
Naramoto, M., Han, Q. & Kakubari, Y. Photosynthetic responses to lightflecks ofFagus crenata seedlings grown in a gap and understory of a deciduous forest. J For Res 7, 193–199 (2002). https://doi.org/10.1007/BF02763132
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02763132