Abstract
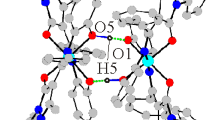
Methods for the synthesis of intracomplex bischelates CuL2, NiL2, CoL2, and the mixed- ligand complex NiL2y2, where L is a deprotonated derivative of the stable nitroxide 4- (2′- hydroxyphenyl)- 2,2,5,5tetramethyl- 3- imidazoline- 1-oxyl, were developed. In the solid state, the compounds have a molecular structure. The most significant difference in the structure of ML2 molecules lies in the values of the angle between the chelate rings (56.9‡ for CuL2, 78.8‡ for CoL2, and 0‡ for NiL2. In complexes with paramagnetic metal ions, the intramolecular exchange interactions are ferromagnetic in character and are close in the order of magnitude (5.7 cm− 1 for CuL2 and 6.8 cm− for NiL2Py2) to the values for complexes with deprotonated enaminoketone derivatives of 3- imidazoline. The NiL2 molecules, having a square coordination of the central atom, are biradicals with antiferromagnetic exchange interactions between the unpaired electrons of nitroxyl groups (− 3.6 cm−). The transformation of NiL2 into NiL2Py2 leads to a transition of Ni(II) from the low- to high- spin state and to ferromagnetic exchange between the unpaired electrons of the paramagnetic centers.
Similar content being viewed by others
References
L. B. Volodarsky, V. A. Reznikov, and V. I. Ovcharenko,Synthetic Chemistry of Stable Nitroxides, CRC Press, Boca Raton, FL (1994).
V. Ovcharenko, A. Burdukov, and R. Musin,Mol. Cryst. Liq. Cryst,273, 89–99 (1995).
G. M. Sheldrick,Acta Crystallogr.,46A, 467–473 (1990).
G. M. Sheldrick,ibid.,49A (Suppl.), C53 (1993).
V. I. Ovcharenko, A. B. Gelman, and V. N. Ikorskii,Zh. Strukt. Khim.,30, No. 5, 142–165 (1989).
V. N. Ikorskii, V. I. Ovcharenko, K. é. Vostrikova, et al.,Zh. Neorg. Khim.,37, 1177–1183 (1992).
L. O. Atovmyan, N. I. Golovina, G. A. Klitskaya, et al.,Zh. Strukt. Khim.,16, No. 4, 624–630 (1975).
V. S. Aliev, A. A. Medzhidov, P. Sh. Mamedova, and Yu. G. Mamedova,Dokl. Akad. Nauk AzSSR, No. 5, 24–28 (1973).
A. A. Medzhidov, T. M. Kutovaya, N. P. Rodin, et al.,Koordinats. Khim.,5, 1431–1439 (1979).
I. A. Timakov, “Synthesis and investigations of the physicochemical properties and reactivities of mono- and binuclear complexes of copper(II) with nitroxides,” Chemical Sciences Candidate’s Dissertation, Baku (1982).
A. A. Medzhidov and I. A. Timakov,Koordinats. Khim.,8, 1043–1049 (1982).
N. P. Rodin, A. A. Medzhidov, T. M. Kutovaya, and M. K. Guseinova,Zh. Strukt. Khim.,22, No. 1, 85–89 (1981).
A. N. Shnulin, Yu. T. Struchkov, Kh. S. Mamedov, et al.,ibid.,18, 1006–1014 (1977).
Author information
Authors and Affiliations
Additional information
Translated fromZhumal Strukturnoi Khimii, Vol. 38, No. 4, pp. 750–761, July–August, 1997.
Rights and permissions
About this article
Cite this article
Ovcharenko, V.I., Fokin, S.V., Romanenko, G.V. et al. Complexes of metals with paramagnetic 3-Imidazoline schiff bases. J Struct Chem 38, 626–636 (1997). https://doi.org/10.1007/BF02762747
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02762747