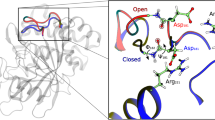
Abstract
In this article we focus on presenting a broad range of examples illustrating low-energy transitions via hinge-bending motions. The examples are divided according to the type of hinge-bending involved; namely, motions involving fragments of the protein chains, hinge-bending motions involving protein domains, and hinge-bending motions between the covalently unconnected subunits. We further make a distinction between allosterically and nonallosterically regulated proteins. These transitions are discussed within the general framework of folding and binding funnels. We propose that the conformers manifesting such swiveling motions are not the outcome of “induced fit” binding mechanism; instead, molecules exist in an ensemble of conformations that are in equilibrium in solution. These ensembles, which populate the bottoms of the funnels,a priori contain both the “open” and the “closed” conformational isomers. Furthermore, we argue that there are no fundamental differences among the physical principles behind the folding and binding funnels. Hence, there is no basic difference between funnels depicting ensembles of conformers of single molecules with fragment, or domain motions, as compared to subunits in multimeric quaternary structures, also showing such conformational transitions. The difference relates only to the size and complexity of the system. The larger the system, the more complex its corresponding fused funnel(s). In particular, funnels associated with allosterically regulated proteins are expected to be more complicated, because allostery is frequently involved with movements between subunits, and consequently is often observed in multichain and multimolecular complexes.
This review centers on the critical role played by flexibility and conformational fluctuations in enzyme activity. Internal motions that extend over different time scales and with different amplitudes are known to be essential for the catalytic cycle. The conformational change observed in enzyme-substrate complexes as compared to the unbound enzyme state, and in particular the hinge-bending motions observed in enzymes with two domains, have a substantial effect on the enzymatic catalytic activity. The examples we review span the lipolytic enzymes that are particularly interesting, owing to their activation at the water-oil interface; an allosterically controlled dehydrogenase (lactate dehydrogenase); a DNA methyltransferase, with a covalently-bound intermediate; large-scale flexible loop motions in a glycolytic enzyme (TIM); domain motion in PGK, an enzyme which is essential in most cells, both for ATP generation in aerobes and for fermentation in anaerobes; adenylate kinase, showing large conformational changes, owing to their need to shield their catalytic centers from water; a calcium-binding protein (calmodulin), involved in a wide range of cellular calcium-dependent signaling; diphtheria toxin, whose large domain motion has been shown to yield “domain swapping” the hexameric glutamate dehydrogenase, which has been studied both in a thermophile and in a mesophile; an allosteric enzyme, showing subunit motion between the R and the T states (aspartate transcarbamoylase), and the historically well-studied lac represoor. Nonallosteric subunit transitions are also addressed with some examples (aspartate receptor andBamHI endonuclease). Hence, using this enzyme-catalysis-centered discussion, we address energy funnel landscapes of large-scale conformational transitions, rather than the faster, quasi-harmonic, thermal fluctuations.
Similar content being viewed by others
References
Fischer, E. (1894)Ber. Dt. Chem. Ges. 27, 2985–2991.
Koshland, D. E., Jr. (1958) Application of a theory of enzyme specificity to protein synthesis.Proc. Natl. Acad. Sci. USA 44, 98–123.
Bryngelson, J. D. and Wolynes, P. G. (1989) Intermediates and barrier crossing in a random energy model (with applications to protein folding).J. Phys. Chem 93, 6902–6915.
Karplus, M. and Shakhnovitch, E. (1992) Protein folding: theoretical studies of thermodynamics and dynamics, inProtein Folding (Creighton T. ed.), W. H. Freeman & Sons, New York, pp. 127–195.
Karplus, M., Sali, A., and Shakhnovitch, E. (1995) Comment: kinetics of protein folding.Nature 373, 664–665.
Wolynes, P. G., Onuchic, J. N., and Thirumalai, D. (1995) Navigating the folding routes.Science 267, 1619–1620.
Onuchic, J. N., Wolynes, P. G., Luthey-Schulten, Z., and Socci, N. D. (1995) Towards an outline of the topography of a realistic protein folding funnel.Proc. Natl. Acad. Sci. USA 92, 3626–3630.
Baldwin, R. L. (1994) Matching speed and stability.Nature 369, 183–184.
Baldwin, R. L. (1995) The nature of protein folding pathways: The classical versus the new view.J. Biomolec. NMR 5, 103–109.
Dill, K. A. and Chan, H. S. (1997) From Levinthal to pathways to funnels.Nature Struct. Biol 4, 10–19.
Karplus, M. (1997) The Levinthal paradox: yesterday and today.Folding Design 2, S69-S75.
Lazaridis, T. and Karplus, M. (1997) “New view” of protein folding reconciled with the old through multiple unfolding simulations.Science 278, 1928–1931.
Gruebele, M. and Wolynes, P. (1998) Satisfying turns in folding transitions.Nature Struct. Biol. 5, 662–665.
Tsai, C.-J., Xu, D., and Nussinov, R. (1998) Protein Folding via Binding, and vice versa.Folding & Design 3, R71-R80.
Gerstein, M., Lesk, A., and Chothia, C. (1994) Structural mechanism for domain movements in proteins.Biochemistry 33, 6739–6749.
Gerstein, M. and Krebs, W. (1998) A database of macromolecular motions.Nucleic Acids Res. 26, 4280–4290.
Derewenda, U., Brzozowski, A. M., Lawson, D. M., and Derewenda, Z. S. (1992) Catalysis at the interface: the anatomy of a conformational change in a triglyceride lipase.Biochemistry 31, 1532–1541.
Brady, L., Brzozowski, A. M., Derewenda, Z. S., Dodson, E., Dodson, G., Tolley, S., et al. (1990) A serine protease triad forms the catalytic centre of a triaclglycerol lipase.Nature 343, 767–770.
Bernstein, F., Koetzle, T., Williams, G., Meyer, E. J., Brice, M., Rodgers, J., et al. (1977) The Protein Data Bank: a computer based archival file for macromolecular structures.J. Mol. Biol. 112, 535–542.
Peters, G. H., Toxvaerd, S., Olsen, O. H., and Svendsen, A. (1997) Computational studies of the activation of lipases and the effect of a hydrophobic environment.Protein Eng. 10, 137–147.
Berg, O. G., Cajal, Y., Butterfoss, G. L., Grey, R. L., Alsina, M. A., Yu, B. Z., and Jain, M. K. (1998) Interfacial activation of triglyceride lipase from Thermomyces (Humicola) lanuginosa: kinetic parameters and a basis for control of the lid.Biochemistry 37, 6615–6627.
Lowe, M. E. (1997) Colipase stabilized the lid domain of pancreatic triglycerate lipase.J Biol. Chem. 272, 9–12.
Iwata, S., Kamata, K., Yoshida, S., Minowa, T., and Ohta, T. (1994) T and R states in the crystals of bacterial L-lactate dehydrogenase reveal the mechanism for allosteric control.Struct. Biol. 1, 176–185
Klimasaukas, S., Kumar, S., Roberts, R. J., and Cheng, X. (1994) HhI methyltransferase flips its target base out of the DNA helix.Cell 76, 357–369.
Sun, J. and Sampson, N. S. (1998) Determination of the amino acid requirements for a protein hinge in triosephosphate isomerase.Protein Sci. 7, 1495–1505.
Mainfroid, V., Terpstra, P., Beauregard, M., Frere, J. M., Mande, S. C., Hol, W. G. J., Martial, J. A., and Goraj, K. (1996) 3 HTIM mutants that provide new insight on why TIM is a dimer.J. Mol. Biol 257, 441–456.
Rietveld, A. W. M. and Ferreira, S. T. (1998) Kinetics and energetics of subunit dissociation/unfolding of TIM: the importance of oligomerization for conformational persistence and chemical stability of proteins.Biochemistry 37, 933–937.
Schliebs, W., Thanki, N., Eritja, R., and Wierenga, R. (1996) Activate-site properties of monomeric triosephosphate isomerse (MONOTIM) as deduced from mutational and structural studies.Protein Sci. 5, 229–239.
Talent, J. M., Zvaigzne, A. I., Agrawal, N., and Gracy, R. W. (1997) Effect of active-site modification on the terminal marking deamidation of triosephosphate isomerase.Arc. Biochem. Biophys 340, 27–35.
Derreumaux, P. and Schlick, T. (1998) The loop opening/closing motion of the enzyme triosephosphate isomerase.Biophys. J. 74, 72–81.
Auerbach, G., Huber, R., Grattinger, M., Zaiss, K., Schurig, H., Jaenicke, R., and Jacob, U. (1997) Closed structure of phosphoglycerate kinase from Thermotoga maritima reveals the catalytic mechanism and determinants of thermal stability.Structure 5, 1475–1483.
Bernstein, B. E., Michels, P. A. M., and Hol, W. G. J. (1997) Synergistic effects of substrate-induced conformational changes in phosphoglycerate kinase activation.Nature 385, 275–278.
Cheung, C. W. and Mas, M. T. (1996) Substrate-induced conformational-changes in yeast 3-phosphoglycerate kinase monitored by fluorescence of single tryptophan probes.Protein Sci. 5, 1144–1149.
Ritcovonsovici, M., Mouratou, B., Minard, P., Desmadril, M., Yon, J. M., Andrieux, M., Leroy, E., and Guittet, E. (1995) Role of the C-terminal helix in the folding and stability of yeast phosphoglycerate kinase.Biochemistry 34, 833–841.
Adams, B., Fowler, R., Hudson, M., and Pain, R. H. (1996) The role of the C-terminal lysine in the hinge bending mechanism of yeast phosphoglycerate kinase.Febs Lett. 385, 101–104.
Pecorari, F., Minard, P., Desmadril, M., and Yon, J. M. (1993) Structure and functional complementation of engineered fragments from yeast phosphoglycerate kinase.Protein Eng. 6, 313–325.
Pecorari, F., Guilbert, C., Minard, P., Desmadril, M., and Yon, J. M. (1996) Folding and functional complementation of engineered fragments from yeast phosphoglycerate kinase.Biochemistry 35, 3465–3476
Parker, M. J., Spencer, J., Jackson, G. S., Burston, S. G., Hosszu, L. L. P., Craven, C. J., Waltho, J. P., and Clarke, A. R. (1996) Domain behavior during the folding of a thermostable phosphoglycerate kinase.Biochemistry 35, 15740–15752.
Sherman, M. A., Beechem, J. M., and Mas, M. T. (1995). Probing intradomain and interdomain conformational-changes during equilibrium unfolding of phosphoglycerate inase-fluorescence and circuilar-dichroism study of tryptophan mutants.Biochemistry 34, 13934–13942.
Lillo, M. P., Szpikowska, B. K., Mas, M. T., Sutin, J. D., Beechem, J. M. (1997) Real-time measurement of multiple intramolecular distances during protein folding reactions: a multisite stopped-flow fluorescence energytransfer study of yeast phosphoglycerate kinase.Biochemistry 36, 11273–11281.
McPhillips, T. M., Hsu, B. T., Sherman, M. A., Mas, M. T., and Rees, D. C. (1996) Structure of the R65Q mutant of yeast 3-phosphoglycerate kinase complexed with Mg-AMP-PNP and 3-phospho-D-glyceratefe.Biochemistry 35, 4118–4127
Schulz, G. E., Muller, C. W., and Diederichs, K. (1990) Induced-fit movements in adenylate kinase.J. Mol. Biol. 213, 627–630.
Schlauderer, G. J., Proba, K., and Schulz, G. E. (1996) Structure of A Mutant Adenylate kinase ligated with an ATP-analog showing domain closure over ATP.J. Mol. Biol. 256, 223–227.
Muller, C. W, Schlauderer, G. J., Reinstein, J., and Schulz, G. E. (1996) Adenylate kinase motion during catalysis: An energetic counter-weight balancing substrate-binding.Structure 4, 147–156.
Zhang, H. J., Sheng, X. R., Pan, X. M., and Zhou, J. M. (1997) Activation of adenylate kinase by denaturants is due to the increasing conformational flexibility at its active sites.Bioch. Biophys. Res. Comm. 238, 382–386.
Ikura, M., Clore, G. M., Gronenborn, A. M., Zhu, G., Klee, C. B., and Bax, A. (1992) Solution structure of a calmodulin-target peptide complex by multidimensional NMR.Science 256, 632–638.
Bennett, M. J., Schlunegger, M. P., and Eisenberg, D. (1995) 3D domain swapping: A mechanism for oligomer assembly.Protein Sci. 4, 2455–2468.
Baker, P. J., Britton, K. L., Engel, P. C., Farrants, G. W., Lilley, K. S., Rice, D. W., and Stillman, T. J. (1992) Subunit assembly and active site location in the structure of glutamate dehydrogenase.Proteins 12, 75–86.
Kumar, S., Tsai, C. J., Ma, B., and Nussinov, R. (1999) Contribution of salt bridges toward protein thermostability.J. Biomolecular Struct. & Dynamics. In press.
Braig, K., Otwinowski, Z., Hegde, R., Boisvert, D. C., Joachimiak, A., Horwich, A. L., and Sigler, P. B. (1994) The crystal structure of the bacterial chaperonin GroEL at 2.8Å.Nature 371, 578–586.
Stevens, R. C., Gouaux, J. E., and Lipscomb, W. N. (1990) Structural consequences of effector binding to the T state of aspartate carbamoyltransferase: crystal structures of the unligated and ATP- and CTP-complexed enzymes at 2.6Å resolution.Biochemistry 29, 7691–7701.
Sakash, J. B. and Kantrowitz, E. R. (1998) The N-terminus of the regulatory chain ofEscherichia coli aspartate transcarbamoylase is important for both nucleotide binding and heterotropic effects.Biochemistry 371, 281–288.
Lewis, M., Chang, G., Horton, N. C., Kercher, M. A., Pace, H. C., Schumacher, M. A., Brennan, R. G., and Lu, P. (1996) Crystal structure of the lactose operon repressor and its complexes with the DNA and inducer.Science 271, 1247–1254.
Milburn, M. V., Prive, G. G., Milligan, D. L., Scott, W. G., Yeh, J., Jancarik, J., Koshland, D. E., and Kim, S.-H. (1991) Three dimensional structures of the ligand-binding domain of the bacterial aspartate receptor with and without a ligand.Science 254, 1342–1347.
Newman, M., Strzelecka, T., Dorner, L. F., Schildkraut, I., and Aggarwal, A. K. (1995) Structure ofBamHI endonuclease bound to DNA: partial folding and unfolding on DNA binding.Science 269, 656–663.
Standfield, R. L., Takimoto-Kanimura, M., Rini, J. M., Profy, A. T., and Wilson, I. A. (1993) Major antigen-induced domain rearrangements in an antibody.Structure 1, 83–93.
Herron, J. N., He, X. M., Ballard, D. W., Blier, P. R., Pace, P. E., Bothwell, A. L., Voss, E. Jr., and Edmundson, A. B. (1991) An autoantibody to single-stranded DNA: comparison of the three-dimensional structures of the unliganded Fab and a deoxynucleotide-Fab complex.Proteins 11, 159–175.
Rini, J. M., Stanfield, R. L., Stura, E. A., Salinas, P. A., Profy, A. T., and Wilson, I. A. (1993) Crystal structure of a human immunodeficiency virus type 1 neutralizing antibody 50.1, in complex with its V3 loop peptide antigen.Proc. Natl. Acad. Sci. USA 90, 6325–6329.
Marmorstein, R. and Harrison, S. C. (1994) Crystal structure of a PPR1-DNA complex: DNA recognition by proteins containing a Zn2Cys6 binuclear cluster.Gen. Dev. 8, 2504–2412.
Marques, O. and Sanejouand, Y. H. (1995) Hinge-bending motion in citrate synthase from normal-mode calculations.Proteins 23, 557–560.
Ikura, T. and Go, N. (1993) Normal-mode analysis of mouse epidermal growth-factor: characterization of the harmonic motion.Proteins 16, 423–436.
Guilbert, C., Pecorari, F., Perahia, D., and Mouawad, L. (1996) Low-frequency motions in phosphoglycerate kinase: a normal mode analysis.J. Chem. Phys. 204, 327–336.
Hayward, S., Kitao, A., and Berendsen, H. J. C. (1997) Model-free methods of analyzing domain motions in proteins from simulation: a comparison of normal mode analysis and molecular dynamics simulation of lysozymeProteins 27, 425–437.
deGroot, B. L., Hayward, S., vanAalten, D. M. F., Amadei, A., and Berendsen, H. J. C. (1998) Domain motions in bacteriophage T4 lysozyme: a comparison between molecular dynamics and crystallographic data.Proteins 31, 116–127.
vanAalten, D. M. F., Grotewold, E., and Joshua Tor, L. (1998) Essential dynamics from NMR clusters: dynamic properties of the Myb DNA-binding domain and a hinge-bending enhancing variant.Methods-A Companion To Methods In Enzymology 14, 318–328.
Alexov, E. and Atanasov, B. (1995) Selective absorption of radio-frequency energy due to collective motion of charged domain: case of lysozyme crystal.J. Biomol. Struct. Dyn. 13, 219–228.
Miura, N., Hayashi, Y., and Mashimo, S. (1996) Hinge-bending deformation of enzyme observed by microwave dielectric measurement.Biopolymer 39, 183–187.
Chandra, N. R., Muirhead, H., Holbrook, J. J., Bernstein, B. E., Hl, W. G. J., and Sessions, R. B. (1998) A general method of domain closure is applied to phosphoglycerate kinase and the result compared with the crystal structure of a closed conformation of the enzyme.Proteins 30, 372–380.
Hayward, S. and Berendsen, H. J. C. (1998) Systematic analysis of domain motions in proteins from conformational change: new results on citrate synthase and T4 lysozyme.Proteins 30, 144–154.
Wriggers, W. and Schulten, K. (1997) Protein domain movements: detection of rigid domains and visualization of hinges in comparisons of atomic coordinates.Proteins 29, 1–14.
Verbitsky, G., Nussinov, R., and Wolfson, H. (1999) Structural comparison allowing hinge bending, swiveling motions.Proteins 34, 232–254.
Bahar, I., Erman, B., Haliloglu, T., and Jernigan, R. L. (1997) Identification of cooperative motions and correlated structural elements in coarse-grained proteins: application to T4 lysozyme.Biochemistry 36, 13512–13523.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, S., Ma, B., Tsai, CJ. et al. Folding funnels and conformational transitions via hinge-bending motions. Cell Biochem Biophys 31, 141–164 (1999). https://doi.org/10.1007/BF02738169
Issue Date:
DOI: https://doi.org/10.1007/BF02738169