Abstract
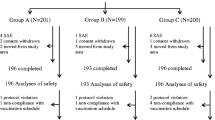
The present prospective, open, controlled, randomised comparative trial was undertaken to evaluate the sero response and side effects of PRP-T Conjugate Vaccine (ACT-HIB) in infants and children aged 2 months and 16–24 months. Fifty four babies aged 2 months formed group A, 56 children aged 16–24 months formed group B. Groups A and B were further subdivided into two sub groups each destined to receive either PRP-T vaccine in association with DPT vaccine at different sites (I) or PRP-T and DPT both vaccines at the same site mixed in the same syringe (II). Group A received 3 doses at 2,3 and 4 months of age and group B received one dose between 16–24 months. The Geometric mean titres of Anti PRP antibodies observed in primary immunisation schedule (A) and single dose vaccination schedule (B) were comparable and significantly higher to prevaccination titres. A serum anti PRP level of > 1.0 mcg/ml after immunisation is believed to correlate with long term protection. Ninety-six percent of infants in Group A and 98% in Group B achieved titres > 1.0 mcg/ml.
The side effects were minimal, local and were comparable between the study and control groups, suggesting that PRP-T vaccine is highly immunogenic and well tolerated in Indian infants and children.
Similar content being viewed by others
References
Fraser DW. Haemophilus influenzae in the community and in the home. In: Sell SH, Wright PF (eds).,Haemophilus influenzae, epidemiology, immunology and prevention of disease. New York : Elsevire, 1982; 11–12.
Dajani AS, Asmar BI, Thirumoorthi MC. Systemic Haemophilus influenzae disease : an overview.J Pediat, 1979; 355–364.
Robbins JB, Schneerson R. Polysaccharide-protein coagulates: a new generation of vaccines.J Infec Dis, 1982; 161 : 321–332.
Eskola J, Peltola H, Takala AHet al. Efficacy of Haemophilus influenzae type b polysaccharide — diphtheria toxoid conjugate vaccine in infancy.N Engl J Med, 1987; 317: 717–722.
Schneerson R, Robbins JB, Parke JC Jr. Quantitative and qualitative analyses of serum antiobodies elicited in adults by H. influenzae type b and pneumococcus type 6A capsular polysaccharide tetanus toxoid conjugates.Infect Immun 1986; 52: 519–528.
Cleasson BA, Schneerson R, Trollfors B, Lagergard T, Taranger J, Robins JB. Duration of serum antibodies elicited by Haemophilus influenzae type b capsular polysaccharide alone or conjugated to tetanus toxoid in 18 to 23 months old children.J Pediatr, 1990; 116: 928–931.
Claesson BA, Schneerson R Robbins JBet al. Protective levels of serum antibodies stimulated in infants by two injections of Haemophilus influenzae type b capsular polysaccharide-tetanus toxoid conjugated.J Pediatr, 1989; 114: 97–100.
Fritzell B, Plotkin S. Efficiency and safety of a Haemophilus influenzae type b capsular polysaccharide-tetanus protein conjugate vaccine.J Pediatr, 1992; 121: 355–362.
Watemberg N, Dagan R, Arbelli Yet al. Safety and immunogenicity of Haemophilus type b-tetanus protein conjugate vaccine, mixed in the same syringe with diphtheria-tetanus-pertussis vaccine in young infants.Infect Dis J, 1991; 10: 758–761.
Barra A, Dagan R, Preud Homme JI, Bajart A, Danve B, Fritzell B. Characterization of the serum antibody response induced by Haemophilus influenzae b tetanus protein-conjugate vaccine in infants receiving a DTP combined vaccine from 2 months of age.Vaccine 1993; 11:1003–1006.
Claesson BA, Trollfors B, Lagergard Tet al. Clinical and immunologic responses to the capsular polysaccharide of haemophilus influenzae type b alone or conjugated to tetanus toxoid in 18 to 23 month old children.J Pediatr. 1988; 112: 695–702.
Booy R, Taylog SA, Dobson SRMet al. Immunogenicity and safety of PRP-T conjugate vaccine given according to the British accelerated immunization schedule.Arch Dis Child, 1992; 67: 475–478.
Decker MD, Edwards KM, Bradley R, Palmer P. Comparative trial in infants of four conjugate Haemophilus influenzae type b vaccine.J Pediatr, 1990; 120: 184–189.
Greenberg DP, Vadheim CM, Marcy MSet al. Evaluation of the safety, immunogenicity and efficacy of Haemophilus inluenzae type b PRP-T conjugate vaccine in a prospective, randomized and placebocontrolled trial in young infants.Program and abstracts of the 31st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, September 29 to October 2,1991; 109.
Granoff DM, Anderson EL, Osterholm MTet al. Differences in the imunogenicity of three Haemophilus influenzae type b conjugate vaccines in infants.J Pediatr (in press).
Ferreccio C, Clemns J, Avendano A,et al. The clinical and immunologic response of Chilean infants to Haemophilus influenzae type b polysaccharide tetanus protein conjugate vaccine coadministered in the same syringe with diphtheria-tetanus toxoid — pertussis vaccine at two, four and six months of age.Pedat Inf Dis J, 1991; 10:764–771.
Fritzell B. Vaccine polyosidique contre Haemophilus influenzae b conjugue a la proteinie tetanique.Immunol Med, 1991; 8:176–183.
Report of the takes force on Pertussis and pertussis Immunisation.Paed, 1988; 81 : 939–984.
Holmes J Sandraet al. Immunogenicity of four Hemophilus influenzae type b conjugate vaccine in 17 to 19 months old children.The Journal of Pediatrics. March 1991; 364–371.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kumar, A., Dutta, A.K., Saili, A. et al. Immunogenicity and tolerance of H. Influenzae Type b, tetanus toxoid conjugate vaccine given concurrently or in combination. Indian J Pediatr 64, 839–847 (1997). https://doi.org/10.1007/BF02725508
Issue Date:
DOI: https://doi.org/10.1007/BF02725508