Abstract
Background: The E1-b attenuated adenovirus, ONYX-015 (Onyx Pharmaceuticals, Richmond, CA), has demonstrated antitumoral activity in patients with recurrent squamous cell carcinoma of the head and neck. This study evaluated the effects of intratumoral ONYX-015 injection combined with systemic chemotherapy.
Methods: Inclusion criteria included: (1) recurrent squamous cell carcinoma of the head and neck, not surgically salvageable, (2) target tumor amenable to direct injection, and (3) no prior chemotherapy for recurrent disease. Patients received ONYX-015 (1010 plaque-forming units) intratumorally for 5 days, cisplatin (80 mg/m2) on day 1, and 5-fluorouracil (800–1000 mg/m2) on days 1–5. This cycle was repeated every 3 weeks. Serial physical examination and computed tomography were used to assess tumor size and treatment response.
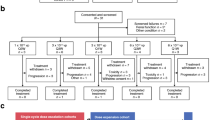
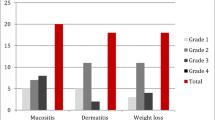
Results: Fourteen patients were enrolled, and nine patients were evaluable for response at the time of enrollment. The mean age of the evaluable patients was 60.8 years (range, 46–71 years). Mean maximum tumor diameter was 4.8 cm (range, 1.9–10.5 cm). Treatment-related toxicity included nausea (n=7, 77.8%), vomiting (n=5, 55.6%), mucositis (n=5, 55.6%), pain at the injection site (n=5, 55.6%), constipation (n=4, 44.4%), and fatigue (n=4, 44.4%). Locoregional tumor control was obtained in all nine patients (100%) (mean observation time, 157 days). Complete clinical response was seen in three patients (33.3%), partial response was seen in three patients (33.3%), minor response was seen in one patient (11.1%), and two patients (22.2%) had stable disease. Median time to local progression of disease has not been reached (range, 35–356 days).
Conclusions: ONYX-015 adenovirus plus systemic cisplatin and 5-fluorouracil provides antitumor activity and local tumor control in patients with recurrent squamous cell carcinoma of the head and neck. This novel treatment approach offers hope for patients with limited treatment alternatives and provides the foundation for a phase III clinical trial.
Similar content being viewed by others
References
Schwartz GJ, Mehta RH, Wenig BL, et al. Salvage treatment for recurrent squamous cell carcinoma of the oral cavity.Head Neck 2000;22:34–41.
Pearlman NW. Treatment outcome in recurrent head and neck cancer.Arch Surg 1979;114:39–42.
Jones KR, Lodge-Rigal RD, Reddick RL, et al. Prognostic factors in the recurrence of stage I and II squamous cell cancer of the oral cavity.Arch Otolaryngol Head Neck Surg 1992;118:483–5.
Fu KK, Lichter A, Galante M. Carcinoma of the floor of the mouth: an analysis of treatment results and the sites and causes of failures.Int J Radiat Oncol Biol Phys 1976;1:829–37.
Paredes J, Hong W, Felder T, et al. Prospective randomized trial of high-dose cisplatin and fluorouracil infusion with or without sodium diethyldithiocaramate in recurrent and/or metastatic squamous cell cancer of the head and neck.J Clin Oncol 1988;6:955–62.
Jacobs C, Lyman G, Velez-Garcia E, et al. A phase III randomized study comparing cisplatin and fluorouracil as single agents and in combination for advanced squamous cell carcinoma of the head and neck.J Clin Oncol 1992;10:257–63.
Forastiere A, Metch B, Schuller D, et al. Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous cell carcinoma of the head and neck: a southwest oncology group study.J Clin Oncol 1992;10:1245–51.
Schrijvers D, Johnson J, Jimenez U, et al. Phase III trial of modulation of cisplatin/fluorouracil chemotherapy by interferon alfa-2b in patients with recurrent or metastatic head and neck cancer.J Clin Oncol 1998;16:1054–9.
Liverpool Head and Neck Oncology Group. A phase III randomized trial of cisplatin, methotrexate, cisplatin + methotrexate, and cisplatin + 5-FU in end stage squamous carcinoma of the head and neck.Br J Cancer 1990;61:311–5.
Clavel M, Vermorken J, Cognetti F, et al. Randomized comparison of cisplatin, methotrexate, bleomycin, and vincristine (CABO) versus cisplatin and 5-fluorouracil versus cisplatin, in recurrent or metastatic squamous cell carcinoma of the head and neck. A phase III study of the EORTC head and neck cancer cooperative group.Ann Oncol 1994;5:521–6.
Boyle JO, Hakim J, Kock WM, et al. The incidence of p53 mutations increases with progression of head and neck cancer.Cancer Res 1993;53:4477–80.
Caamano J, Zhang SY, Rosvold EA, et al. P53 alterations in human squamous cell carcinomas and carcinoma cell lines.Am J Pathol 1993;142:1131–9.
Shin DM, Lee JS, Lippman SM, et al. p53 expression: predicting recurrence and second primary tumors in head and neck squamous cell carcinoma.J Natl Cancer Inst 1996;8:519–28.
Sauter ER, Ridge JA, Litwin S, et al. Pretreatment p53 protein expression correlates with decreased survival in patients with end-stage head and neck cancer.Clin Cancer Res 1995;1:1407–12.
Debbas M, White E. Wild type p53 mediates apoptosis by E1A, which is inhibited by E1B.Genes Dev 1993;7:547–54.
Grand RJ, Grant ML, Gallimore PH. Enhanced expression of p53 in human cells infected with metastatic adenoviruses.Virology 1994;203:229–40.
Lowe SW, Ruley HE. Stabilization of the p53 tumor suppression is induced by adenovirus 5E1A and accompanies apoptosis.Genes Dev 1993;7:535–40.
Heise CC, Williams A, Olesch J, et al. Efficacy of a replication competent adenovirus (ONYX-015) following intratumoral injection: intratumoral spread and distant effects.Cancer Gen Ther 1999;6:499–504.
Heise CC, Sampson-Johannes A, Williams A, et al. ONYX-015, and E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents.Nat Med 1997;6:639–45.
Bischoff JR, Kirn DH, Williams A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells.Science 1996;274:373–6.
Khuri F, Nemunaitis J, Ganly I, et al. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-FU in patients with recurrent head and neck cancer.Nat Med (in press).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lamont, J.P., Nemunaitis, J., Kuhn, J.A. et al. A prospective phase II trial of ONYX-015 adenovirus and chemotherapy in recurrent squamous cell carcinoma of the head and neck (the baylor experience). Ann Surg Oncol 7, 588–592 (2000). https://doi.org/10.1007/BF02725338
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02725338