Abstract
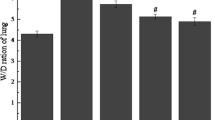
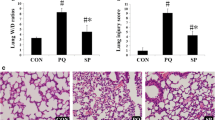
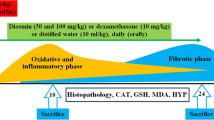
Paraquat toxicity often presents as acute lung injury that progresses into the irreversible form of the adult respiratory distress syndrome (ARDS). To assess the role of inflammatory cells in paraquat toxicity and its modulation by endotoxin/hyperoxia pretreatment, rats were injected with paraquat (35 mg/kg i.p.) and injury monitored by histologic study and bronchoalveolar lavage. Forty-eight hours after paraquat administration, acute lung injury was confirmed by histologic study and was associated with an increase in lavage LDH activity from 0.37 ± 0.12 U/mg protein to 0.83 ± 0.22 U/mg protein (P < 0.05). Lavage revealed a decrease in alveolar macrophages, 95.6 ± 0.6 to 20.6 ± 3.4%, and an increase in neutrophils from 1.6 ± 0.2 to 70.6 ± 6.1% (P < 0.001 in both comparisons). Pretreatment of rats with endotoxin and hyperoxia prior to paraquat administration prolonged survival (P < 0.005), elevated endogenous superoxide dismutase and catalase levels in the lung (P < 0.005, both comparisons), and significantly reduced the inflammatory cell changes (P < 0.001). The protective role of this pretreatment regimen in paraquat toxicity may reflect a complex modulating effect on several parameters critical to the pathogenesis of paraquat-induced lung injury.
Similar content being viewed by others
References
Aerts C, Voisin C (1981) In vitro toxicity of oxygen and oxygen-paraquat association on alveolar macrophages surviving in gas phase. Bull Eur Physiopathol Respir 17:145–151
Aldrich TK, Fisher AB, Cadenas E, Chance B (1983) Evidence for lipid peroxidation by paraquat in the perfused rat lung. J Lab Clin Med 101:66–73
Babior BM, Kipnes RS, Curnutte JT (1973) Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest 52:741–744
Bawdey JA, Karnovsky ML (1980) Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem 49:695–726
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Boyden S (1962) The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leukocytes. J Exp Med 115:453–466
Bus JS, Aust SD, Gibson JE (1974) Superoxide and singlet oxygen catalyzed lipid peroxidation as a possible mechanism for paraquat (methyl viologen) toxicity. Biochem Biophys Res Commun 58:749–755
Flick MR, Perel A, Staub NC (1981) Leukocytes are required for increased lung microvascular permeability after microembolization in sheep. Circ Res 48:344–351
Forman HJ, Aldrich TK, Posner MA, Fisher AB (1982) Differential paraquat uptake and redox kinetics of rat granular pneumocytes and alveolar macrophages. J Pharmacol Exp Ther 221:428–433
Fox RB, Hoidal JR, Brown DM, Repine JE (1980) Mechanism of pulmonary oxygen toxicity: hyperoxia damaged alveolar macrophages (AM) release factors which attract polymorphonuclear leukocytes (PMA) and stimulate the release of superoxide (O 2− ). Clin Res 28:528A
Fox RB, Hoidal JR, Brown DM, Repine JE (1981) Pulmonary inflammation due to oxygen toxicity: involvement of chemotactic factors and polymorphonuclear leukocytes. Am Rev Respir Dis 123:521–523
Frank L (1981) Prolonged survival after paraquat. Role of the lung antioxidant enzyme systems. Biochem Pharmacol 30:2319–2324
Gadek JE, Hunninghake GW, Zimmerman RL, Crystal RG (1980) Regulation of the release of alveolar macrophage-derived neutrophil chemotactic factor. Am Rev Respir Dis 121:723–733
Hammerschmidt DE, Weaver LJ, Hudson LD, Craddock PR, Jacob HS (1980) Association of complement activation and elevated plasma-C5a with adult respiratory distress syndrome. Lancet 1:947–949
Hammerschmidt DE, Harris PD, Wayland JH, Craddock PR, Jacobs HS (1981) Complement-induced granulocyte aggregation in vivo. Am J Pathol 102:145–150
Henson PM, Larsen GL, Webster RO, Mitchell BC, Goins AJ, Henson JE (1982) Pulmonary microvascular alterations and injury induced by complement fragments: synergistic effect of complement activation, neutrophil sequestration and prostaglandins. Ann NY Acad Sci 384:287–300
Hosea S, Brown E, Hammer C, Frank M (1980) Role of complement activation in a model of adult respiratory distress syndrome. J Clin Invest 66:375–382
Klebanoff SJ (1975) Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol 12:117–142
Mantel N (1966) Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50:163–170
Martin WJ, II Gadek JE, Hunninghake GW, Crystal RG (1981) Oxidant injury of lung parenchymal cells. J Clin Invest 68:1277–1288
Martin WJ II (1984) Neutrophils kill pumonary endothelial cells by a H2O2 dependent pathway: an in vitro model of the adult respiratory distress syndrome. Am Rev Respir Dis 130:209–213
McCord JM and Fridovich I (1969) Superoxide dismutase: an enzymatic function for erythrocuprein hemocuprein. J Biol Chem 244:6049–6055
Misra HP, Gorsky LD (1981) Paraquat and NADPH-dependent lipid peroxidation in lung microsomes. J Biol Chem 256:9994–9998
Repine JE, White JG, Clawson CC, Holmes BM (1974) Effects of phorbol myristate acetate on the metabolism and ultrastructure of neutrophils in chronic granulomatous disease. J Clin Invest 54:83–90
Richmond R, Halliwell B (1982) Formation of hydroxyl radicals from the paraquat radical cation, demonstrated by a highly specific gas chromatographic technique. The role of superoxide radical anion, hydrogen peroxide and glutathione reductase. J Inorg Biochem 17:95–107
Roth RA (1981) Effect of pneumotoxicants on lactate dehydrogenase activity in airways of rats. Tox Appl Pharmacol 57:69–78
Ryan GB, Hurley JV (1966) The chemotaxis of polymorphonuclear leukocytes towards damaged tissue. Br J Exp Pathol 47:530–536
Sachs T, Moldow CF, Craddock PR, Bowers TK, Jacobs HS (1978) Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. J Clin Invest 61:1161–1167
Schlag G, Voight WH, Redl H, Glatzl A (1980) Vergleischende morphologie des posttraumatischen lungenversagens. Anasth Intensivther Notfallmed 15:315–339
Schoenberger CI, Rennard SI, Bitterman PB, Fukuda Y, Ferrans VJ, Crystal RG (1984) Paraquat-induced pulmonary fibrosis. Role of the alveolities in modulating the development of fibrosis. Am Rev Respir Dis 129:168–173
Shasby DM, Fox RB, Harada RN, Repine JE (1982) Reduction of the edema of acute hyperoxic lung injury by granulocyte depletion. J Appl Physiol 52:1237–1244
Smith LL, Rose MS, Wyatt I (1978) The pathology and biochemistry of paraquat. Ciba Found Symp 65:321–341
Suttorp N, Simon LM (1982) Lung cell oxidatn injury. J Clin Invest 70:342–350
Synderman R, Phillips JK, Mergenhagen SE (1971) Biological activity of complement in vivo: role of C5 in the accumulation of polymorphonuclear leukocytes in inflammatory exudates. J Exp Med 134:1131–1143
Thet LA, Wrobel DJ, Crapo JD, Shelburne JD (1983) Morphologic aspects of the protection by endotoxin against acute and chronic oxygen-induced lung injury of adult rats. Lab Invest 48:448–457
Turner SR, Tainer JA, Lynn WS (1975) Polymorphonuclear leukocyte chemotaxis toward oxidized lipid components of cell membranes. J Exp Med 141:1437–1441
Weiss SJ, Young J, LoBuglio AF, Slivka A, Nimek NF (1981) Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest 68:714–721
Wroblewski F, LaDue JS (1955) Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med 90:210–213
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Martin, W.J., Howard, D.M. Paraquat-induced neutrophil alveolitis: Reduction of the inflammatory response by pretreatment with endotoxin and hyperoxia. Lung 164, 107–120 (1986). https://doi.org/10.1007/BF02713633
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02713633