Abstract
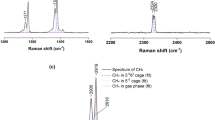
13C NMR spectra were obtained for pure CH4, mixed CH4+THF, and mixed CH4+Neohexane hydrates in order to identify hydrate structure and cage occupancy of guest molecules. In contrast to the pure CH4 hydrates, the NMR spectra of the mixed CH4+THF hydrate verified that methane molecules could occupy only the small portion of 512 cages because the addition of THF, water-soluble guest component, to aqueous solution prevents the complete filling of methane molecules into small cages. Furthermore, from these NMR results one important conclusion can be made that methane molecules can’t be enclathrated at all in the large 51264 cages of structure II. In addition, gas uptake measurements were carried out to determine methane amount consumed during pure and mixed hydrate formation process. The moles of methane captured into pure CH4 hydrate per mole of water were found to be similar to the full occupancy value, while the moles of methane captured into the mixed CH4+THF hydrate per moles of water were much lower than the ideal value. The overall results drawn from this study can be usefully applied to storage and transportation of natural gas.
Similar content being viewed by others
References
Adisasmito, S., Frank, R. J. and Sloan Jr., E. D.,“Hydrates of Carbon Dioxide and Methane Mixtures”J. Client. Eng. Data,36, 68 (1991).
Englezos, P.,“Clathrate Hydrates,”Ind. Eng. Chem. Res.,31, 2232 (1992).
Gudmundsson, J. S., Parlaktuna, M. and Khokhar, A. A.,“Storing Natural Gas as Frozen Hydrate,” SPE Production and Facilities, February, 69 (1994).
Gudmundsson, J. S., Mork, M. and Graff, O. F.,“Hydrate Non-Pipeline Technology,” Proceedings of the 4th International Conference on Gas Hydrates, Yokohama, 997 (2002).
Holder, G. D., Zeets, S. P. and Pradhan, N.,“Phase Behavior in Systems Containing Clathrate Hydrates,”Rev. Chem. Eng.,5, 1 (1988).
Kang, S.-R, Seo, Y.-T, Ryu, B.-J. and Lee, H.,“S{H} Hydrate Equilibria of (Methane+Water+2-Methylbutane+MgCl2), (Methane+Water+ 2,2-Dimethylbutane+MgCl2), and (Methane+Water+Methylcyclohexane+MgC2,”J. Chem. Thermodynamics,31, 763 (1999).
Khokhar, A. A., Gudmundsson, J. S. and Sloan Jr., E. D.,“Gas Storage in Structure H Hydrates,”Fluid Phase Equilibria,150-151, 383 (1998).
Lee, J.-W, Chun, M.-K, Lee, K.-M, Kim, Y.-J. and Lee, H,“Phase Equilibria and Kinetic Behavior of CO2 Hydrate in Electrolyte and Porous Media Solutions: Application to Ocean Sequestration of CO2,”Korean J.Chem. Eng.,19, 673 (2002).
Lundgaard, L. and Mollerup, J. M.,“The Influence of Gas Phase Fugaclty and Solubility on Correlation of Gas-Hydrate Formation Pressure,”Fluid Phase Equilibria,70, 70 (1991).
Parrish, W. R. and Prausnitz, J.M.,“Dissociation Pressures of Gas Hydrates Formed by Gas Mixtures,”AIChEJ.,11, 26 (1972).
Reid, R C, Prausmtz, J. M. and Poling, B. E.,“The Properties of Gases and Liquids,” 4th edition, McGraw-Hill, New York (1987).
Ripmeester, J. A. and Ratcliffe, C. I.,“Low-Temperature Cross-Polarization/Magic Angle Spinning13C NMR of Solid Methane Hydrates: Structure, Cage Occupancy, and Hydrauon Number,”J. Phys. Chem.,92, 337 (1988).
Ripmeester, J. A. and Ratcliffe, C. I.,“The Diverse Nature of Dodecahedral Cages in Clathrate Hydrates As Revealed by129Xe and13C NMR Spectroscopy: CO2 as a Small-Cage Guest,”Energy & Fuel,12, 197 (1998).
Seo, Y. and Lee, H.,“A New Hydrate-Based Recovery Process for Removing Chlorinated Hydrocarbons from Aqueous Solutions,”Environ. Sci. Technol.,35, 3386 (2001).
Seo, Y.-T, Kang, S.-R, Lee, H, Lee, C.-S. and Sung, W.-M, “Hydrate Phase Equilibria for Gas Mixtures Containing Carbon Dioxide: A Proof-of-concept to Carbon Dioxide Recovery from Multicomponent Gas Stream,”Korean J. Chem. Eng.,17, 659 (2000).
Seo, Y.-T, Kang, S.-P. and Lee, H.,“Experimental Determination and Thermodynamic Modeling of Methane and Nitrogen Hydrates in the presence of THF, Propylene Oxide, 1,4-Dioxane and Acetone,”Fluid Phase Equilibria,189, 99 (2001).
Seo, Y.-T. and Lee, H.,“Multiple-Phase Hydrae Equilibria of the Ternary Carbon Dioxide, Methane, and Water Mixtures,”J. Phys. Chem. B,105, 10084 (2001).
Sloan, E. D.,“Clathrate Hydrates of Natural Gases 2nd Edition,” Marcel Dekker, New York (1998).
Subramaman, S. and Sloan Jr., E. D., “Molecular Measurements of Methane Hydrate Formation,”Fluid Phase Equilibria,58-160, 813 (1999).
Subramaman, S., Kim, R A., Dec, S. F. and Sloan Jr., E. D., “Evidence of Structure II Hydrate Formation from Methane+Ethane Mixtures,”Chem. Eng Sci.,55, 1981 (2000).
De Swaan Arons, J. and Diepen, G. A.,Rec. Trav. Chim.,82, 249 (1963).
Udachin, K. A. and Ripmeester, J. A.,“A Complex Clathrate Hydrate Structure Showing Bimodal Guest Hdration,”Nature,397, 420 (1999).
van der Waals, J. H. and Platteeuw, J. C, “Clathrate Solutions,”Adv. Chem. Phys.,2, 1 (1959).
Zhong, Y and Rogers, R. E., “Surfactant Effects on Gas Hydrate Formation,”Chem. Eng. Sci.,55, 4175 (2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seo, YT., Lee, H. 13C NMR analysis and gas uptake measurements of pure and mixed gas hydrates: Development of natural gas transport and storage method using gas hydrate. Korean J. Chem. Eng. 20, 1085–1091 (2003). https://doi.org/10.1007/BF02706941
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02706941