Abstract
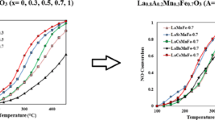
In the present work, we have investigated the reduction of NO by propane over perovskite-type oxides prepared by malic acid method. The catalysts were modified to enhance the activity by substitution of metal into A or B site of perovskite oxides. In addition, the reaction conditions, such as temperature, O2 concentration, and space velocity have been varied to understand their effects on the catalytic performance. In the LaCoO3 type catalyst, the partial substitution of Ba and Sr into A site enhanced the catalytic activity in the reduction of NO. For the La0.6Ba (Sr)o.4 Co1−x FexO3 (x=0-1.0) catalyst, the partial substitution of Fe into B site enhanced the conversion of NO, but excess amount of Fe decreased the conversion of NO. The surface area and catalytic activity of perovskite catalysts prepared by malic acid method showed higher values than those of solid reaction method. The conversion of NO increased with increasing O2 concentration and contact time. The introduction of water into reactant feed decreased the catalytic activity but the deactivation was shown to be reversible over La0.6Ba0.4Co1−x ,FexO3 catalyst.
Similar content being viewed by others
References
Armor, J. N., “Catalytic Removal of Nitrogen Oxides: Where are the Opportunities?”,Catal. Today,26, 99 (1995).
Anderson, D. J. and Sale, F. R., “Production of Conducting Oxide Powders by Armophous Citrate Process”,Powder Metall,2L, 14 (1979).
Cook, R. L. and Sammuells, A. F.,Solid State Chenu,14, 395 (1975).
Iwamoto, M., Furikawa, H., Mine, Y., Uemura, F., Mikuriya, S. and Kagawa, S.,J. Chem. Soc, Chem. Commun., 1272 (1986).
Iwamoto, M. and Hamada, H.,Catal. Today,10, 57 (1991).
Iwamoto, M. and Yahiro, H.,Catal. Today,22, 5 (1994).
Jonker, G. H. and Van Santen, J. H.,Physica,19, 120 (1953).
Li, Y. and Armor, J. N., “Selective Catalytic Reduction of NO, with Methane over Metal Exchanged Zeolites“,Appl Catal. B,2, 239 (1993).
Libby, W. F., “Promising Catalyst for AutoExhaust”,Science,171, 499 (1971).
Monroe, D. R., Dimaggio, C. L., Beck, D. D. and Matekunas, F. A., SAE 930737 (1993).
Montreuil, C. N. and Shelef, M.,Appl. Catal., B,1, LI (1992).
Moon, H. D. and Lee, H.-I., “A Study on the Catalytic Characteristics of Oxygen Reduction in the Alkaline Fuel Cell “,J.Kor. Ind. & Eng. Chem.,7(3), 554 (1996).
Nam, I. S., “A Catalytic Process for the Reduction of NO, from Stationary Sources”,Catalysis,11, 5 (1995).
Obayashi, H. and Kudo, T.,Jpn. J. AppL Phys.,14, 330 (1975).
Sato, S., Yu, Y., Yahiro, H., Mizuno and Iwamoto, M., “Cu-ZSM-5 Zeolite as Highly Active Catalyst for Removal of Nitrogen Monoxide from Emission of Diesel Engines”,Appl. Catal.,70, LI (1991).
Shangguan, W. F., Teraoka, Y. and Kagawa, S., “Simultaneous Catalytic Removal of NOx and Diesel Soot Particulates over Ternary AB2O4 Spinel-type Oxides”,Appl. Catal., B,8, 217 (1996).
Teraoka, Y., Hakebayzshi, H., Moriguchi, I. and Kagawa, S., “Oxygen-Sorptive Properties and Direct Structure of Perovskite-Type Oxide”,Chem. Lett., 673 (1991).
Torikai, Y., Yahiro, H., Mizuno, N. and Iwamoto, M.,Catal. Lett.,9, 91 (1991).
Voorhoeve, R. J. H., Remeika, J. P. and Trimble, L. E., “Perovskites Containing Ruthenium as Catalysts for Nitric Oxide Reduction”,Ann, N.Y. Acad. Sci.,272, 3 (1976).
Yao, H. C. and Shelef, M., “Surface Interaction of Oxygen and Nitric Oxide with Mangneous Oxide”,J.Catal.,31, 377 (1973).
Zhang, X., Waletrs, A. B. and Vannice, M. A., “NO Decomposition and Reduction by Methane over La2O3”,Appl. Catal, B,4, 237(1994).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hong, SS., Lee, GD., Park, JW. et al. Catalytic reduction of no over perovskite-type catalysts. Korean J. Chem. Eng. 14, 491–497 (1997). https://doi.org/10.1007/BF02706598
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02706598