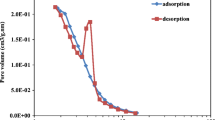
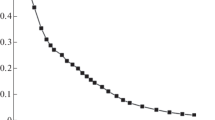
Abstract
The adsorption characteristics of 2,4-dinitrophenol from water onto a granular activated carbon, F-400, were studied at pH 4.3, 7 and 10. Adsorption equilibria of 2,4-dinitrophenol on GAC could be represented by Sips equation. Equilibrium capacity increased with decreasing pH. The differences in the rates of adsorption are primarily attributable to the differences in the equilibrium at the various pHs. Intraparticle diffusion was explained by surface diffusion mechanism. An adsorption model based on the linear driving force approximation (LDFA) was used for simulating the adsorption behavior of 2,4-dinitrophenol in a fixed bed adsorber.
Similar content being viewed by others
Abbreviations
- As :
-
surface area of the sorbent particles [m2]
- c:
-
concentration in the fluid phase [mol/m3]
- ci :
-
initial concentration of bulk fluid [mol/m3]
- cs :
-
concentration on the surface of adsorbent [mol/m3]
- DL :
-
axial dispersion coefficient [m2/sec]
- Dm :
-
molecular diffusion coefficient [m2/sec]
- Dp :
-
effective pore diffusion coefficient [m2/sec]
- Ds :
-
effective surface diffusion coefficient [m2Vsec]
- L:
-
bed length [m]
- kf :
-
film mass transfer coefficient [m/sec]
- M:
-
total mass of sorbent particle
- NA :
-
rate of mass transfer of adsorbates to the external surface of the adsorbent [mol/sec]
- q:
-
equilibrium amount adsorbed on the adsorbent [mol/kg]
- r:
-
radial distance [m]
- Rp :
-
particle radius [m]
- t:
-
time [sec, hr]
- V:
-
volume of solution [m3]
- v:
-
interstitial velocity [m/sec]
- vs :
-
superficial velocity [m/sec]
- z:
-
axial distance [m]
- εb :
-
bed porosity [-]
- ρp :
-
particle density [kg/m3]
- GAC:
-
granular activated carbon
- MTZ:
-
mass transfer zone
- PDM:
-
pore diffusion model
- Re:
-
Reynolds number
- Sc:
-
Schmidt number
- SDM:
-
surface diffusion model
References
Kamel, D. and Tahar, S., “Kinetics of Heterogeneous Photocatalytic Decomposition of 2,4-Dichlorophenoxyacetic Acid over Titanium Dioxide and Zinc Oxide in Aqueous Solution”,Pestic. Sci.,54, 269 (1998).
Mehmet, M., Irfanet, K. and Melda, T., “Removal of 2,4-D from Aqueous Solution by the Adsorbents from Spent Bleaching Earth”,J. Environ. Sci. Health,B35(2), 187 (2000).
Misic, D. M., Sudo, Y., Suzuki, M. and Kawazeo, K., “Liqiid to Particle Mass Transfer in a Stirred Batch Adsorptiom Tank with Nonlinear Isotherm”,J. Chem, Eng. Japan,15, 490 (1982).
Moon, H. and Lee, W. K., “Intraparticle Diffusion in Liquid Phase Adsorption of Phenols with Activated Carbon in Finite Batch Adsorber”,J. of Colloid and Interface Sci.,96, 162 (1983).
Moon, H. and Tien, C., “Further Work om Multicomponent Adsorption Equilibria Calculation Based on the ideal Adsorbed Solution Theory”,Ind. Eng. Chem. Res.,26, 2024 (1987).
Reid, R. C., Prausnitz, J.M. and Pokung, B. E., “The Properties of Gases and Liquides”, McGraw-Hill Co., New York (1994).
Ruthven, D. M., “Principles of Adsorption and Adsorption Processes”, John Wiley and Sons, New York (1984).
Teng, H. and Hsieh, C., “Influence of Surface Characteristics on Liquid-phase Adsorption of Phenol by Activated Carbons Prepared from Bituminous Coal”,Ind. Eng. Chem. Res.,37, 3618 (1998).
Wakao, N. and Funazkri., “Effect of Fluid Dispersion Coefficient on Particle to Fluid Mass Transfer Coefficients in Packed Bed”,Chem. Eng. Sci.,33, 1375 (1978).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, T.Y., Kim, S.J. & Cho, S.Y. Effect of pH on adsorption of 2,4-dinitrophenol onto an activated carbon. Korean J. Chem. Eng. 18, 755–760 (2001). https://doi.org/10.1007/BF02706396
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02706396