Abstract
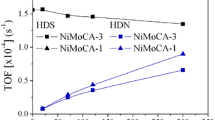
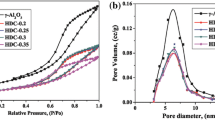
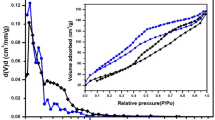
The hydrocracking and hydrodesulfurization (HDS) of n-heptane containing 0.2 mole% dibenzothiophene (DBT) were performed simultaneously using NiPtMo catalysts supported on HZSM-5, LaY and γ-Al2O3 in a high pressure fixed bed reactor. Molybdenum played an important role in both hydrocracking and hydrodesulfurization (HDS). We found that the sulfur compound, dibenzothiophene (DBT). in the reactant was adsorbed on a molybdenum site and converted to hydrogen sulfide so that the active sites of the catalysts for hydrocracking were less poisoned by DBT and the conversion of n-heptane over molybdenum impregnated catalyst was higher than that over molybdenum-free catalyst. The crystal structures of the molybdenum supported on the zeolite and γ-Al2O3 were mainly MoO2.5 (OH)0.5[021] and MoO3[210] respectively as shown by XRD analysis. The structure of MoO2.5(OH)0.5 was easily reduced to MoS2[003] during the reaction. After the reaction of 100 hours over the catalyst supported on γ-Al2O3 the crystal structure of MoO3[210] partially changed to MoO3[300] and the structure of MoS2[003] was not observed. Because of the reactant shape selectivity of zeolite, the acid and the metal sites in the intracrystalline of the catalysts supported on zeolites were less poisoned by DBT. Therefore, both hydrocracking and HDS using n-heptane containing 0.2 mole% of DBT were successfully demonstrated over the prepared catalysts.
Similar content being viewed by others
References
Abbot, J. and Wojciechowski, B. W., “Catalytic Reactions of Branched Paraffins on HY Zeolite”,J. Catal.,113, 353(1988).
Abbot, J., “Cracking Reactions of C6 Paraffins on ZSM-5”,Appl. Catal.,57, 105(1990).
Cerveny, L., “Catalytic Hydrogenation”, 1st ed., Elsevier, N.Y., 150 (1986).
Cscsery, S. M., “ACS Monograph”,171, 680(1974).
Fahrentort et al., “The Mechanism and Heterogeneous Catalysis”. Elsevier, Amsterdam, 25–80 (1982).
Heinemann, H., Mills, G. A., Miliken, J. H. and Oblad, A. G., “Catalytic Mechanism”,Ind. Eng. Chem.,45, 134 (1953).
Hilfman, L, “Hydrocracking Catalyst”, U.S. Patents 4. 141, 759, April 10(1979).
Imlik, B., Naccache, C, Coudurier, G., Praliaud, H., Meriaudeau, P., Gallezot, P., Martin, G. A., Verdrine, J. C, “Metal-Supported and Metal-Additive Effects in Catalysis”, Elsevier, N.Y., 247 (1982).
Kirchen, R. P., Sorensen, T. S., Wagstaff, K. and Walker, A.M., “The Characterization of and the Evolution of Molecular Hydrogen by the μ-Hydride-bridged Cycrodecyl Cation”,Tetrahedron,42(4), 1063(1986).
Mikovsky, R. J. and Marshall, J. F., “Random Aluminum-Ion Siting in the Faujasite Lattice”,J. Catal,44, 170(1976).
O’hara, M. J.Johnson, R. W., Hoffman, E., Hilfman, L., “Hydrocracking Catalyst”. U.S. Patents4, 141, 860, Feb. 27(1979).
Olah, G. A., Halpern, Y., Shen, J. and Mo, Y. K., “Electrophilic Reaction at Single Bonds. III. Hydrogen-Deutrium Exchange and Protolysis(Deuterolysis) of Alkanes with Superacids”,J. Amer. Chem. Soc.,93, 1251(1971).
Sinfelt, J. H.,Adv. Chem. Eng..5, 37 (1964).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, MC., Kim, KL. A role of molybdenum and shape selectivity of catalysts in simultaneous reactions of hydrocracking and hydrodesulfurization. Korean J. Chem. Eng. 13, 1–6 (1996). https://doi.org/10.1007/BF02705881
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02705881