Abstract
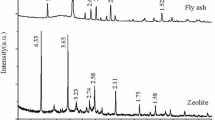
Power plants burning coal generate a large amount of fly ash as waste matter. The objective of this study is to produce zeolitic adsorbents that possesses high adsorptive capacity for toxic cations. The sample was first pretreated with a High Intensity Magnetic Separator for the removal of iron and magnetic materials (mainly Fe2O3 and TiO2). The zeolitic adsorbents were prepared under the various conditions of NaOH concentration (1–5 N), reaction time from 3 to 96 hours and at the various temperatures of 60, 80 and 100°C. The results of the experiment showed that the coal fly ash should be synthesized with 4 N NaOH for 48 hours at 100°C in order to have good adsorptive capacity. The zeolitic adsorbents showed higher cation exchange capacity values than the natural zeolite in removing NH +4 , Pb2+, Ca2+and Cd2+ions.
Similar content being viewed by others
References
Barrer, R. M., “Hydrothemal Chemistry of Zeolites”, Academic Press, (1982).
Break, D. W., “Zeolite Molecular Sieves”, John Wiley & Sons Inc., (1974).
Fanor, M., Fabio, R., Ligia, S., Jaime, E., Jose, R. and Fernandez, “New Perspectives for Coal Ash Utilization: Synthesis of Zeolitic Materials”,Fuel,69, 263 (1990).
Fisher, G. L. and Natusch, D. F. S., “In Analytical Methods for Coal and Coal Products”, Academic Press, N.Y., USA, 3, 489 (1978).
Falcone, S. K., Schobert, H. H., Rindt, D. K. and Braun, S.,Am. Chem. Soc. Div. Fuel Chem. Prepr.,29(4), 76 (1984).
Hara, N. and Takahashi, “Zeolite: Introduction and Application”, Tokyo, Japan, 300 (1975).
Jorgensen, S. E. and Barkaes, K., “Ammonium Removal by Use of Clinoptilolite”,Water Research,10, 213 (1974).
Munson, R. A. and Sheppard, R. A., “Natural Zeolite: Their Properties, Occurrences and Uses”,Minerals Sci. Eng.,6(1), 19 (1974).
Natush, D. F. S. and Taylor, D. R., “Environmental Effects of Western Coal Combution: Part IV”, Environ. Research Lab., Duluth, Mn, USA, (1980).
Sand, L. B., “Synthesis or Large-port and Small-port Mordenite”, Molecular Sieves, London, Soc. Chem. Ind., 71 (1968).
Shepard, A. O. and Starkey, H. C, “Effect on Cation Exchange on the Thermal Behavior of Heulandite and Clinoptilolite”, U.S. Geol. Survey Prof. 475-D, 89 (1963).
Shin, B. S., Choi, K. S. and Kim, J. U., “A Study on the Removal of Ammonia in Waste Water by Zeolite Mineral”,J of the Kor. Inst, of Mineral & Mining Eng.,24(3), 202 (1987).
Shin, B.S., Jung, C.J., Choi, S.J., Oh, J. G. and Choi, K. S., “A Study on the Removal of Heavy Metal Ions from Waste Water by Natural Zeolites”,J. of the Kor. Inst, of Mineral & Mining Eng,19(2), 143 (1982).
Szostak, R., “Molecular Sieves-Principles of Synthesis and Identification”, Van Nostrano Reinhold, (1989).
Wills, B. A., “Mineral Processing Technology”, Pergamon Press, 4, (1988).
Haruna, J., “Synthesis of Zeolite from Fly Ash”, Japan Patent, 90-229709, (1990).
Clean Japan Center, “Introduction of a Demonstration Plant for the Production of Zeolite from Fly Ash”, (1991).
Korea Coal Industry Promotion Board, “A Study on the Support System of the Government for Inducement of Facility Investment on Coal Mining”, (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shin, BS., Lee, SO. & Kook, NP. Preparation of zeolitic adsorbents from waste coal fly ash. Korean J. Chem. Eng. 12, 352–357 (1995). https://doi.org/10.1007/BF02705768
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02705768