Abstract
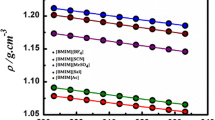
The surface tension and viscosity of 1-butyl-3-methylimidazolium iodide ([bmim][I]) and 1-butyl-3-methylimidazolium tetrafluoroborate ([bmim][BF4]) were measured over a temperature range of 298. 15 to 323.15 K. It was found that both the viscosity and surface tension decrease with increasing temperature and that the surface tension and viscosity values of [bmim][I] were higher than those of [bmim][BF4]. Additionally, the solubility of lithium bromide (2)+1-butyl-3-methylimidazolium bromide ([bmim][Br]) (3) in water (1) was measured at three different mass ratios (w2/w3=4 and 7, w3=0) by using a visual polythermal method. The solubility of the suggested systems was better than that of lithium bromide in water.
Similar content being viewed by others
References
Kim, J.-S. and Lee, H., “Solubilities, vapor pressures, densities, and viscosities of the LiBr+LiI+HO(CH2)3OH+H2O system,”J. Chem. Eng. Data,46, 79 (2001).
Kim, J.-S., Park, Y. and Lee, H., “Solubilities and vapor pressures of the water+lithium bromide+ethanolamine system,”J. Chem. Eng. Data,41, 279 (1996).
Kim, K.-S. and Lee, H., “Densities, viscosities, and surface tensions of thetrifluoroethanol+quinoline system,”J. Chem. Eng. Data,47, 216 (2002a).
Kim, K.-S., Choi, S., Demberelnyamba, D., Lee, H., Oh, J., Lee, B.-B. and Mun, S.-J., “Ionic liquids based on N-alkyl-N-methylmorpho-linium salts as potential electrolytes,”Chem. Commun., 828 (2004a).
Kim, K.-S., Park, S.-Y., Choi, S. and Lee, H., “Vapor pressures of the 1-butyl-3-methylimidazolium bromide+water, 1-butyl-3-methylimi-dazolium tetrafluoroborate+water, and 1-(2-hydroxyethyl)-3-meth-ylimidazoliumtetrafluoroborate+water systems,”Chem. Eng. Data,49, 1550 (2004c).
Kim, K.-S., Shin, B.-K. and Lee, H., “Physical and electrochemical properties of 1-butyl-3-methylimidazolium bromide, 1-butyl-3-methylimi-dazolium iodide, and 1-butyl-3-methylimidazolium tetrafluoroborate,”Korean J. Chem. Eng.,21, 1010 (2004d).
Kim, K.-S., Shin, B.-K., Lee, H. and Ziegler, F., “Refractive index and heat capacity of 1-butyl-3-methylimidazolium bromide and 1-butyl-3-methylimidazolium tetrafluoroborate, and vapor pressure of binary systems for 1-butyl-3-methylimidazolium bromide+trifluoroethanol and 1-butyl-3-methylimidazolium tetrafluoroborate+trifluoroethanol,”Fluid Phase Equilibr.,218, 215 (2004b).
Marsh, K. N., Deev, A., Wu, A. C.-T., Tran, E. and Klamt, A., “Room temperature ionic liquids as replacements for conventional solvents — a review,”KoreanJ. Chem. Eng.,19, 357 (2002).
Park, Y., Kim, J.-S. and Lee, H., “Density, vapor pressure, solubility, and viscosity for water+lithium bromide+lithium nitrate+1,3-propanediol,”J. Chem. Eng. Data,42, 145 (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, KS., Demberelnyamba, D., Shin, BK. et al. Surface tension and viscosity of 1-butyl-3-methylimidazolium iodide and 1-butyl-3-methylimidazolium tetrafluoroborate, and solubility of lithium bromide+1-butyl-3-methylimidazolium bromide in water. Korean J. Chem. Eng. 23, 113–116 (2006). https://doi.org/10.1007/BF02705701
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02705701