Abstract
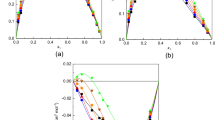
We present experimental data of speed of sound, refractive index on mixing and density for the binaries of propyl acetate+(toluene, ethylbenzene, p-xylene, isopropylbenzene, butylbenzene, isobutylbenzene, mesitylene, or t-butylbenzene) at T=298.15 K and 1 atm, and the corresponding computed derived magnitudes (change of isentropic compressibility, change of refractive index on mixing, and excess molar volume). The mixtures show a clear expansive tendency for the highest molar weight compounds, and the steric hindrance role of the aromatic chemicals was analyzed to the light of the non ideality on mixing. Values of physical properties were compared with the results obtained by theoretical estimation procedures.
Similar content being viewed by others
References
Bevington, P.,Data reduction and error analysis for the physical sciences, McGraw-Hill, New York (1969).
Kay, W. E., “Density of hydrocarbon gases and vapors at high temperature and pressure,”J. Ind. Eng. Chem.,28, 1014 (1936).
Iglesias, M., Orge, B. and Tojo, J., “Refractive indices, densities and excess properties on mixing of the systems acetone+methanol+water and acetone+methanol+1-butanol at 298.15 K,”Fluid Phase Eq.,126, 203(1996).
Nutsch-kuhnkies, R., “Sound characteristics in binary mixtures and solutions,”Acustica,15(1), 383 (1965).
Prausnitz, J. M. and Gunn, R. D., “Volumetric properties of nonpolar gaseous mixtures,”AIChE J.,4, 430 (1958).
Qin, A., Hoffman, D. E. and Munk, P.,J. Chem. Eng. Data,37, 66 (1992).
Redlich, O. and Kister, A. T., “Thermodynamics properties and the classification of solutions,”Ind. Eng. Chem.,40, 345 (1948).
Reid, R. C., Prausnitz, J. M. and Poling, B. E.,The properties of gases and liquids, 4th ed., McGraw-Hill, Singapore (1988).
Resa, J. M., Gonzalez, C., Lanz, J. and Mtz. de Ilarduya, J. A., “Excess volumes for binary mixtures formed by methyl acetate with aromatic hydrocarbons,”J. of Thermal Anal. and Calorimetry,52(3), 895 (1998).
Resa, J. M., Gonzalez, C., Iglesias, M., Lanz, J. and Mtz de Ilarduya, J. A., “Excess volumes of binary mixtures of vinyl acetate and aromatic hydrocarbons,”J. Chem. Thermodyn.,33(7), 723 (2001).
Resa, J. M., Gonzalez, C., Ortiz de Landaluce, S. and Lanz, J., “Densities, excess molar volumes, and refractive indices of ethyl acetate and aromatic hydrocarbon binary mixtures,”J. Chem. Thermodyn.,34(7), 995 (2002).
Riddick, J. A., Bunger, W. B. and Sakano, T. K.,Organic solvents, Wiley-Interscience, USA (1986).
Schaaffs, W., “Problem of a theoretical calculation of the velocity of sound for binary liquid mixtures,”Acustica,33(4), 272 (1975).
Spencer, C. F. and Danner, R. P., “Improved equation for prediction of saturated liquid density,”J. Chem. Eng. Data,17(2), 236 (1972).
TRC thermodynamic tables, Thermodynamic Research Center, Texas A&M University: College Station, TX (1994).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Resa, J.M., González, C., Prieto, S. et al. Mixing properties of propyl acetate+aromatic hydrocarbons at 298.15 K. Korean J. Chem. Eng. 23, 93–101 (2006). https://doi.org/10.1007/BF02705698
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02705698