Abstract
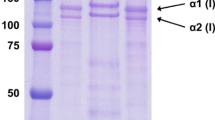
The muscle collagen of marine prawn,Penaeus indicus, was isolated by limited pepsin digestion. Based on selective salt precipitation, amino acid composition and gel electrophoretic pattern, the major collagen was found to be a homotrimer of á 1 chain, similar to type V collagen of vertebrates. Electron microscopy of reconstituted fibrils, made for the first time from a crustacean species, revealed a characteristic 64 nm periodicity. Biochemical studies indicate a less than normal amount of associated carbohydrates and an increased alanine content The major collagen had a denatu ration temperature of 37°C with an intrinsic viscosity of 11.3 dl/g. Spectral characteristics of the major collagen were studied. Results suggest the presence of genetically distinct collagen types and acid resistant cross links in crustacean muscle.
Similar content being viewed by others
References
Bailey A J, Shells well G B and Duance V C 1979 Identification and change of collagen types in differentiating myoblasts and developing chick muscle;Nature (London)278 67–68
Birk D E, Fitch J M, Babiarz J P and Linsenmeyer T F 1988 Collagen type I and type V are present in the same fibril in the corneal stroma;J. Cell. Biol. 106 999–1004
Broek D L, Madri J, Eikenberry E F and Brodsky B 1995 Characterization of the tissue form of type V collagen from chick bone;J. Biol. Chem. 260 555–562
Dubois M, Gilles K A, Hamilton J K, Rebers P A and Smith F 1956 Colorimetric method for determination of sugars and related substances;Anal. Chem. 28 350–356
Elson L A and Morgan W T J 1933 A colorimetric method for the determination of glucosamine and chondrosamine;Biochem. J. 27 1824–1828
Fessler L I, Kumamoto C A, Meis M E and Fessler J H 1981 Assembly and processing of procollagen V (AB) in chick blood vessels and other tissues;J. Biol. Chem. 256 9640–9645
Gaille F 1985Biology of Invertebrate and lower vertebrate collagens (New York: Plenum Press)
Jackson M, Choo L P, Watson P H, Halliday W C and Mantsch H H 1995 Beware of connective tissue proteins: Assignment and implications of collagen absorption in infrared spectra of human tissues;Biochim. Biophys. Acta 1270 1–6
Kimura S and Tanaka H 1986 Partial Characterisation of muscle collagens from prawns and lobsters;J. Food Sci. 51330–332
Laemmli U K 1970 Cleavage of structural proteins during the assembly of the head of bacteriophage T4;Nature (London)227 680–685
Light N and Champion A E 1984 Characterisation of muscle epimysium, perimysium and endomysium collagens;Biochem. J. 219 1017–1026
Lowry O H, Rosebrough N J, Farr A L and Randall R J 1951 Protein measurement with the folin phenol reagent;J. Biol. Chem. 193 265–276
Moradi-Ameli M, Rousseau J C, Kleman J P, Champliaud M F, Boutillon M M, Bernillon J, Wallach J and Vander Rest M 1994 Diversity in the processing events at the N-terminus of type V collagen;Eur. J. Biochem. 221 987–995
Na G C 1988 UV Spectroscopic characterisation of type I collagen;Coll. Relat. Res. 8 315–330
Rigby B J 1967 Relation between the shrinkage of native collagen in acid solution and the melting temperature of the tropocollagen molecule;Biochim. Biophys. Acta 133 272–277 141
Sato K, Yoshinaka R, Sato M and Shimizu Y 1986 Collagen content in the muscle of fishes in association with their swimming movement and meat texture;Bull. Jpn. Soc. Sci. Fish 52 1595–1600
Sato K, Yoshinaka R, Sato M and Shimizu Y 1987 Isolation of native acid soluble collagen from fish muscle;Nippon Suisan Gakkaishi 53 1431–1436
Sato K, Yoshinaka R, Sato M, Itoh Y and Shimizu Y 1988 Isolation of types I and V collagens from carp muscle;Comp. Biochem. Physiol. B90 155–158
Sato K, Yoshinaka R, Sato M and Tomita J 1989a Biochemical characterisation of collagen in myocommata and endomysium fractions of carp and spotted mackerel muscle;J. Food Sci. 53 1511–1514
Sato K, Yoshinaka R, Itoh Y and Sato M 1989b Molecular species of collagen in the intramuscular connective tissue offish;Comp. Biochem. Physiol. B92 87–91
Sato K, Ohashi C, Ohtsuki K and Kawabata M 1991 Type V collagen in trout (Salmo gairdneri) muscle and its solubility change during chilled storage of muscle;J. Agric. Food. Chem. 39 1222–1225
Van der Rest M, Foucher E A, Dublet B, Eichenberger D, Font Band Goldschmidt D 1991 Structure and function of the Fibril associated collagens;Biochem. Soc. Trans. 19 820–824
Wetlaufer D B 1962 Ultraviolet spectra of proteins and amino acids;Adv. Prot. Chem. 17 330–390
Woessner J F Jr 1961 The determination of hydroxyproline in tissue and protein samples containing small proportions of this amino acid;Arch. Biochem. Biophys. 93 440–447
Yoshinaka R, Sato K, Itoh Y, Nakajima S and Sato M 1989 Content and partial characterisation of collagen in crustacean muscle;Comp. Biochem. Physiol. B94 219–223
Yoshinaka R, Mizuta S, Itoh Y and Sato M 1990 Two genetically distinct types of collagen in kuruma prawn,Penaeusjaponicus;Comp. Biochem. Physiol. B96 451–456
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sivakumar, P., Suguna, L. & Chandrakasan, G. Purification and partial characterization of a type V like collagen from the muscle of marine prawn,Penaeus indicus . J. Biosci. 22, 131–141 (1997). https://doi.org/10.1007/BF02704727
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02704727