Abstract
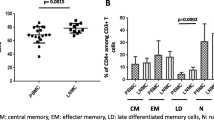
Interferon-(IFN-γ) has been considered to be a critical protective immunomodulatory component against Mycobacterium tuberculosis (M. tb.) infection. In this study T-cell proliferation and IFN-γ production upon stimulation with M. tb. were assessed in patients of pulmonary tuberculosis and healthy contacts. The studies were based on lymphocyte transformation test and detection of intracellular IFN-γ production by CD4 + ve T-cells by flowcytometry. Patients showed lower levels of proliferation, the stimulation index being in the range of 2.17 1.1 (mean + SD) compared to the contacts (SI = 4 59±1.6) (P < 0.01). The kinetics of intracellular induction of IFN-γ on M. tb. stimulation showed a proportional increase in the CD4 + ve T-cell population. The increase was maximal between 96–120 h of culture. In healthy contacts the number of IFN-γ expressing CD + ve T-cells increased to 2.5 to 41 × 104 cells/ml in M. tb. stimulated cultures compared to control cultures (0.1 – 15 × 104). In contrast patients showed no/marginal increase in CD4 + ve T-cell population expressing intracellular IFN-γ Thus the lack of induction of IFN in CD4 + ve T-cells in patients could be a critical shortcoming in their ability to combat tubercle bacilli infection.
Similar content being viewed by others
Abbreviations
- M. tb:
-
Mycobacterium tuberculosis (H37Rv) IFN γ interferon-γ
- LTT:
-
lymphocyte transformation test
- S.I:
-
stimulation index
- PBS:
-
phosphate buffer saline
- DPBS:
-
Dulbecco’s phosphate buffer saline
- PFA:
-
paraformaldehyde
- FACS:
-
fluorescence activated cell sorter
- PHA:
-
phytohaemaglutinin
- PBMC:
-
peripheral blood mononuclear cell
- BD:
-
Becton Dickinson
- FCS:
-
fetal calf serum
- SA-PE:
-
streptavidin-phycoerythrin
- FITC:
-
fluorescein isothiocyanate
References
Assenmacher M, Schmitz J and Radruch A 1994 Flowcytometric determination of cytokines in activated murine T helper lymphocytes: expression of interleukin-10 in interferon- and interleukin-4 expressing cells;Eur. J. Immunol. 24 1097–1101
Bancroft G J, Schreiber R D and Unanue E R 1991 Natural immunity: a T-cell-independent pathway of macrophage activation defined in scid mouse;Immunol. Rev. 124 5–24
Barnes P F, Lu S, Abrams J S, Wang E, Yamamura M and Modlin R L 1993 Cytokine production at the site of disease in human tuberculosis;Infect. Immun. 61 3482–3489
Boyum A 1968 Isolation of mononuclear cells and granulocytes from human blood;Scand. J. Clin. Lab. Invest. (Suppl. 97) 21 77–89
Chan J, Xing Y, Magliozzo R S and Bloom B R 1992 Killing of virulentMycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages;J. Exp. Med. 175 1111–1122
Cooper A M, Dalton D K, Stewart T A, Giffin J P and Orme IM 1993 Disseminated tuberculosis in interferon-τ gene-disrupted mice;J. Exp. Med. 178 2243–2247
Curry R C, Kiener P K and Spitalny G L 1987 A sensitive immunochemical assay for biologically active mu IFN-τ; J. Immunol. Methods104 137–142
Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A and Bloom B R 1993 An essential role for IFN-t in resistance toMycobacterium tuberculosis infection;J. Exp Med. 178 2249–2254
Hahn H and Kaufmann SHE 1981 Role of cell mediated immunity in bacterial infections;Rev. Infect. Dis. 3 1221–1250
Harlow E and Lane D 1988 Labeling antibodies with biotin; inAntibodies: A Laboratory Manual (New York: Cold Spring Harbor Laboratory) pp 340–341
Johnson B J and McMurray D N 1994 Cytokine gene expression by cultures of human lymphocytes with autologousMycobacterium tuberculosis - infected monocytes;Infect. Immun. 62 1444–1450
Jung T, Schauer U, Heusser C, Neumann C and Riegar C 1993 Detection of intracellular cytokines by flowcytometry;J. Immunol. Methods 159 197–207
Kamijo R, Le J, Shapiro D, Havell E A, Huang S, Aguet M, Bosland M and Vilcek J 1993 Mice that lack the interferon τ receptor have profoundly altered responses to infection withBacillus calmette -Guerin and subsequent challenge with lipopolysaccharide;J. Exp. Med. 178 1435–1440
Munk M E, Gatrill A J and Kaufmann SHE 1990 Antigen-specific target cell lysis and interleukin-2 secretion byMycobacterium tuberculosis-activated τ/δ T cells;J. Immunol. 145 2434–2439
Orme I M 1987 The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection withMycobacterium tuberculosis;J. Immunol. 138 293–298
Orme I A, Miller E S, Roberts A D, Furney S K, Grifin J P, Dobos K M, Chi D, Rivoire B and Brennan P J 1992 T lymphocytes mediating protection and cellular cytolysis during the course ofM. tuberculosis infection. Evidence for diferent kinetics and recognition of a wide spectrum of protein antigens;J. Immunol. 148 189–196
Onwubalili J K, Scott G M and Robinson J A 1985 Deficient immune interferon production in tuberculosis;Clin. Exp. Immunol. 59 405–413
Rook G A W, Steele J, Ainswarth M and Champion B R 1986 Activation of macrophages to inhibit proliferation ofMycobacterium tuberculosis: comparison of the effects of recombinant gamma interferon on human monocytes and murine peritoneal macrophages;Immunology 59 333–338
Sanchez F O, Rodriguez J I, Agudelo G and Garcia L F 1994 Immune responsiveness and lymphokine production in patients with tuberculosis and healthy controls;Infect. Immun. 62 5673–5678
Sander B, Andersson J and Andersson U 1991 Assessment of cytokines by immunofluorescence and the paraformaldehyde — saponin procedure;Immunol. Rev. 119 65–93
Saunders B M and Cheers C 1995 Inflammatory response following intranasal infection withMycobacterium avium complex: Role of T cell subsets and gamma interferon;Infect. Immun. 63 2282–2287
Surcel H M, Troye-Blomberg M, Paulie S, Andersson G, Moreno C, Pasvol G and Ivanyi J 1994 T H1/T H2 profiles in tuberculosis, based on the proliferation and cytokine response of blood lymphocytes to mycobacterial antigens;Immunology 81 171–176
Zhang M, Lin Y, Iyer D V, Gong J, Abrams J S and Barnes P F 1995 T cell cytokine responses in human infection with Mycobacterium tuberculosis;63 3231–3234
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhattacharyya, S., Das, S.N., Dey, A.B. et al. Flowcytometric assessment of intracellular interferon -γ production in human CD4 + ve T-cells on mycobacterial antigen stimulation. J. Biosci. 22, 99–109 (1997). https://doi.org/10.1007/BF02703622
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02703622