Abstract
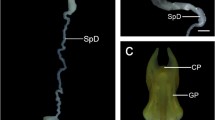
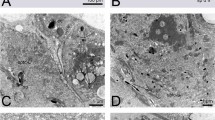
Females of phlebotomine sandflies (Diptera: Psychodidae) possess highly variable spermathecae that present several important taxonomic characters. The cause of this diversity remains a neglected field of sandfly biology, but may possibly be due to female post-mating sexual selection. To understand this diversity, a detailed study of the structure and function of the spermathecal complex in at least one of the species was a prerequisite. Using scanning and transmission electron microscopy, described here is ultrastructure of the spermathecal complex in the sand fly,Phlebotomus papatasi Scopoli. The spermathecal complexes are paired; each consists of a long spermathecal duct, a cylindrical spermathecal body, and a spherical spermathecal gland. Muscle fibres, nerves, tracheoles, and vascular sinuses connect the spermathecal body and duct through the epithelial layers. Spermathecal gland is formed by a typical insect epidermis and consisting of an epithelial layer of class-1 epidermal cells and elaborate glandular cells of class-3 epidermal cells, each having both receiving and conducting ductules (i.e. “end apparatus”) and a “cytological apodeme”, which is a newly described cell structure. The spermathecal body and duct are lined by class-1 epidermal cells and a cuticle, and are enveloped by a super-contracting visceral muscular system. The cuticle consists of rubber-like resilin, and its fibrillar arrangement and chemical nature are described. A well-developed neuromuscular junction exists between the spermathecal gland and the spermathecal body, which are connected to each other by a nerve and a muscle. The spermathecal complexes of the sandfly are compared with those of other insect species. The physiological role and possible evolutionary significance of the different parts of spermathecal complex in the sandfly are inferred from the morphology and behaviour. Post-mating sexual selection may be responsible for the structural uniqueness of the spermathecal complex in phlebotomine sandflies.
Similar content being viewed by others
References
Arnqvist G M, Edvardsson U and Friberg Nilsson T 2000 Sexual conflict promotes speciation in insects;Proc. Natl. Acad. Sci. USA 97 10460–10464
Clements A N and Potter S A 1967 The fine structure of spermathecae and their ducts in the mosquito,Aedes aegypti;J. Insect Physiol. 13 1825–1836
Dallai R 1975 Fine structure of the spermatheca ofApis mellifera;J. Insect Physiol. 21 89–109
Davey K G and Webster G F 1967 The structure and secretion of the spermatheca ofRhodnius prolixus Stal: a histochemical study;Can. J. Zool. 45 653–657
Degrugillier M E and Leopold R 1976 Ultrastructure of sperm penetration of house fly eggs,Musca domestica;J. Ultrastruct. Res. 56 312–325
Eberhard W G 1996Female control: sexual selection by cryptic female choice (Princeton: Priceton University Press)
Eberhard W G 1998 Female roles in sperm competition; inSperm competition and sexual selection (eds) T R Birkhead and A P Moller (London: Academic Press) pp 91–116
Edler H Y 1975 Muscle structure; inInsect muscle (ed.) P N R Usherwood (London: Academic Press) pp 1–65
Filosi M and Perotti M E 1975 Fine structure of the spermatheca ofDrosophila melanogaster Meig;J. Submicrosc. Cytolol. 7 259–270
Filshie B K 1982 Fine structure of the cuticle of insects and other arthropods; inInsect ultrastrucutre (eds) R C King and H Akai (New York: Plenum Press) vol. 1, pp 281–312
Fristrom D K and Rickoll W L 1982 The morphogenesis of imaginal discs ofDrosophila; inInsect ultrastructure (eds) R C King and H Akai (New York: Plenum Press) vol. 1, pp 341–367
Fritz A and Turner F R 2002 A light and electron microscopic study of the spermathecae and ventral receptacle ofAnastrepha suspense (Diptera: Tephritidae) and implications in female influence of sperm storage;Arthrop. Struct. Dev. 30 292–313
Grodner M L 1978 Fine structure of the spermathecal gland of the cotton boll weevil,Anthonomus grandis Boheman (Coleoptera: Curculionidae);Int. J. Insect Morphol. Embryol. 8 51–58
Gupta B L and Smith D S 1969 Fine structural organisation of the spermathecae in the cockroachPeriplaneta americana;Tissue Cell 1 295–324
Happ G M and Happ C M 1970 Fine structure and histochemistry of the spermathecal gland in the meal worm beetle,Tenebrio molitor;Tissue Cell 2 443–446
Holland B and Rice W R 1998 Chase-away sexual selection: antagonistc seduction versus resistance;Evolution 52 1–7
Ilango K 2005 Structure and function of the spermathecal complex in the phlebotomine sandflyPhlebotomus papatasi Scopoli (Diptera: Psychodidae): II. Post-copulatory histophysiological changes during the gonotrophic cycle;J. Biosic. 30 733–747
Ilango K 2004 Phylogeny of the Old World Phlebotomine sand flies (Diptera: Psychodidae) with special reference to structural diversity of female spermathecae;Oriental Insects 38 419–462
Ilango K and Lane R P 2000 Coadaptaion of male aedeagal filaments and female spermthecal ducts of the Old World Phlebotomine sand flies (Diptera: Psychodidae);J. Med. Entomol. 37 653–659
Jones C J and Fischman D A 1970 An electron microscope study of the spermathecal complex of virginAedes aegypti mosquitoes;J. Morphol. 12 293–312
Kokwaro D E, Odiambo T R and Murthi J K 1981 Ultrastructural and histochemical study of the spermatheca of the tsetse,Glossina morsitans moristans;Insect Sci. Appl. 2 135–143
Miya K 1982 Early embryogenesis ofBombyx mori; inInsect ultrastructure (eds) R C King and H Akai (New York: Plenum press) vol. 2, pp 49–77
Neville A C 1970 Cuticle ultrastructure in relation to the whole insect; inInsect ultrastructure (ed.) A C Neville (Symposia of the Royal Entomological Society of London) vol. 5, pp 17–39
Noirot C and Quennedey A 1974 Fine structure of insect epidermal glands;Annu. Rev. Entomol. 19 61–80
Noirot C and Quennedey A 1991 Glands, gland cells, glandular units: Some comments on terminology and classification;Ann. Soc. Entomol. Fran. 27 123–128
Osborne M P 1975 The ultrastrucutre of nerve-muscle synapses; inInsect muscle (ed.) P N R Usherwood (London: Academic Press) pp 151–174
Parker G A 1970 Sperm competition and its evolutionary consequences in the insects;Biol. Rev. 45 525–567
Pitnick S, Markow T and Spicer G S 1999 Evolution of multiple kinds of female sperm-storage organs inDrosophila;Evolution 53 1804–1822
Pitnick S and Brown W D 2000 Criteria for demonstrating female sperm choice;Evolution 54 1052–1056
Rice M J 1970 Supercontracting and non-supercontrating visceral muscles in the tsetse fly,Glossina austeni;J. Insect Physiol. 16 1109–1122
Ridely M 1989 The incidedence of sperm displacement in insects: four conjectures, one corroboration;Biol. J. Linn. Soc. 38 349–367
Rudall K M 1969 Chitin and its association with other molecules;J. Polym. Sci. Part C 28 83–102
Sareen M L, Gill A and Biswas S 1989 Electron microscopic and histochemical studies on the spermatheca of a syrphid fly,Eristalis tenax (Diptera: Syrphidae);Proc. Indian Natl. Sci. Acad. 55 97–102
Satir P and Gilula N B 1973 The fine structure of membranes and intercellular communication in insects;Annu. Rev. Entomol. 18 143–166
Theodor O 1965 On the classification American Phlebotominae;J. Med. Entomol. 2 171–199
Tombes A S 1976 Myoneural junctions and neurosecretory endings on spermathecal muscle fibers of two weevils,Sitophilus granarius andHypera postica;J. Insect Physiol. 22 1573–1580
Tombes A S and Roppel RM 1971 Scanning electron microscopy of the spermatheca inSitophilus granaries;Tissue Cell 3 551–556
Tombes A S and Roppel R M 1972 Ultrastructure of spermatheca of the granary weevil,Sitophilus granarius (Coeoptera: Curculionidae);Int. J. Insect Morphol. Embryol. 1 141–152
Villavaso E J 1975a Function of the spermathecal muscle of the boll weevilAnthonomus grandis;J. Insect Physiol. 21 1275–1278
Villavaso E J 1975b The role of the spermathecal gland of the boll weevilAnthonomus grandis;J. Insect Physiol. 21 1457–1462
Walker W F 1980 Sperm utilization strategies in nonsocial insects;Am. Nat. 115 780–799
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ilango, K. Structure and function of the spermathecal complex in the phlebotomine sandflyPhlebotomus papatasi Scopoli (Diptera: Psychodidae): I. Ultrastructure and histology. J. Biosci. 30, 711–731 (2005). https://doi.org/10.1007/BF02703571
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02703571