Abstract
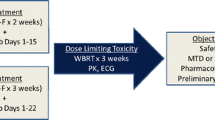
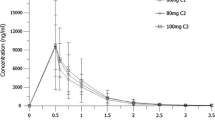
A phase I/II trial was conducted to determine the toxicities and efficacy (overall response, overall survival, and progression-free survival) of the combination of topotecan and whole brain radiation therapy (XRT) in patients with brain metastases. Patients received 30 Gy XRT given in 10 fractions to the whole brain. In phase I, patients were treated in groups of three at each topotecan dose level; dose escalation proceeded until the maximum tolerated dose (MTD) was identified. The dose-limiting toxicity proved to be grade IV neutropenia at 0.6 mg/m2/d, resulting in an MTD of 0.5 mg/m2/d. One of nine patients showed a response to treatment, and that was partial (OR 11%). Three had stable disease (33%), and four experienced progressive disease (44%). Median progression-free survival was 60 d; median overall survival was 102 d. Intravenous topotecan at 0.5 mg/m2/d concomitant to XRT with 30 Gy in 3-Gy fractions is tolerable in patients with brain metastases. This regimen has the additional advantage of providing systemic treatment to patients with metastases in other locations while whole brain radiation is in progress. Although response and survival outcomes in this small study do not appear higher than expected from historical controls, these were not primary end points, and larger studies on this topic would be useful to elucidate the efficacy of this combination treatment regimen.
Similar content being viewed by others
References
DeVita VT, Rosenberg SA, Hellman S.Cancer: Principles and Practices of Oncology. Lippincott-Raven: Philadelphia, PA, 1997.
Pollock BE. Management of patients with multiple brain metastases.Contemp Neurosurg 1999;21: 1–6.
Lock RB, Ross WE. DNA topotecanisomerases in cancer therapy.Anticancer Drug Design 1987;2: 151–164.
Friedman HS, et al. Activity of 9-dimethylaminomethyl-10-hydroxycamptothecin against pediatric and adult central nervous system tumor xenografts.Cancer Chemother Pharmacol 1994;34: 171–174.
Blaney SM, et al. Plasma and cerebrospinal fluid pharmacokinetic study of Topotecan in nonhuman primates.Cancer Res 1993;53: 725–727.
Ardizzoni A, et al. Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: a phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative Group.J Clin Oncol 1997;15 (Suppl. 5): 2090–2096.
Macdonald, DR, et al. NCIC-CTG phase II study of topotecan in malignant glioma.J NeuroOncology 1996;28: 72.
Fisher BJ, Scott C, Macdonald DR, Coughlin C, Curran WJ. Phase I study of topotecan plus cranial radiation for glioblastoma multiforme: results of Radiation Therapy Oncology Group Trial 9507.J Clin Oncol 2001;19(4): 1111–1117.
Fisher, BJ, Won M, Macdonald D, Johnson DW, Roa W. Phase II study of topotecan plus cranial radiation for glioblastoma multiforme: results of Radiation Therapy Oncology Group 9513.Int J Radiat Oncol Biol Phus 2002;53(4): 980–986.
Chen AY, Choy H, Rothenbery ML. DNA topotecanisomerase I-targeting drugs as radiation sensitizers.Oncology (Huntingt) 1990;13 (Suppl. 10): 39–46.
Personal communication: Three patients were treated using this protocol at Medical University of South Carolina; however, these data were not available for review and analysis of these patients is not included in this publication.
Hochster H, et al. Activity and pharmacodynamics of 21-day topotecan infusion in patients with ovarian cancer previously treated with platinum-based chemotherapy. New York Gynecologic Oncology Group.J Clin Oncol 1999;17(8): 2553–2561.
Antonadou D, et al. Phase II randomized trial of temozolomide and concurrent radiotherapy in patients with brain metastases.J Clin Oncol 2002;20: 3644–3650.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mirmiran, A., McClay, E. & Spear, M.A. Phase I/II study of IV topotecan in combination with whole brain radiation for the treatment of brain metastases. Med Oncol 24, 147–153 (2007). https://doi.org/10.1007/BF02698033
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02698033