Abstract
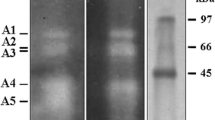
Considering the fact that Prays oleae is one of the most pathogenic insects to the olive tree in the Mediterranean particularly in Tunisia, the mode of action of Cry insecticidal toxins of Bacillus thuringiensis kurstaki in Prays oleae midgut was investigated. The proteolysis of Bacillus thuringiensis δ-endotoxins in the midgut was a key step in determining their potency against Prays oleae. The latter's proteases activated the δ-endotoxins early, yielding stable toxins. The in vitro and in vivo binding of these toxins to Prays oleae larvae midgut was studied immunohistochemically, evidencing a midgut columnar cell vacuolization, microvilli damage, and then a pass of epithelium cell content into the larvae midgut. Moreover, Bacillus thuringiensis toxins were shown to bind to the apical microvilli of the midgut epithelial cells. The in vitro study of the interaction of Prays oleae midgut proteins with biotinylated Bacillus thuringiensis toxins allowed the prediction of four suitable receptor proteins in Prays oleae.
Similar content being viewed by others
References
Arambourg, Y. (1986) Traité d'entomologie oléicole. Conseil oléicole international, Madrid.
Schnepf, E., Crickmore, N., Van Rie, J., et al. (1998) Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62, 775–806.
Höfte, H. and Whiteley, H. R. (1989) Insecticidal crystal protein of Bacillus thuringiensis. Microbiol. Rev. 53, 242–255.
Tounsi, S., Dammak, M., Rebai, A., and Jaoua, S. (2005) Response of larval Ephestia kuehniella (Lepidoptera/Pyralidae) to individual Bacillus thuringiensis kurstaki toxins and toxin mixtures. Biol. Control. 35. 27–31.
De Maagd, R. A., Bravo, A., and Crickmore, N. (2001) How Bacillus thuringiensis has evolved specific toxins to colonize the insect world? Trends Genet. 17, 193–199.
Knight, P. J. K., Crickmore, N., and Ellar, D. J. (1994) The receptor for Bacillus thuringiensis Cry 1A(c) delta-endotoxin in the brush border membrane of the lepidopteran Manduca sexta is aminopeptidase N. Mol. Microbiol. 11, 429–436.
Gill, S. S., Cowles, E. A., and Francis, V. (1995) Identification, isolation and cloning of a Bacillus thuringiensis Cry 1Ac toxin-binding protein from the midgut of the lepidopteran insect Heliothis virescens. J. Biol. Chem. 270, 27,277–27,282.
Jaoua, S., Zouari, N., Tounsi, S., and Ellouz, R. (1996) Study of d-endotoxins produced by three recently isolated strains of Bacillus thuringiensis. FEMS Microbiol. Lett. 145, 349–354.
Tounsi, S., J'Mal, A., Zouari, N., and Jaoua, S. (1999) Cloning and nucleotide sequence of a novel cry 1Aa-type gene from Bacillus thuringiensis subsp. kurstaki. Biotechnol. Lett. 21, 771–775.
Tounsi, S., Dammak, M., Zouari, N., Rebai, A., and Jaoua, S. (2006) Evidence of the effect of δ-endotoxin ration in Bacillus thuringiensis crystals on the toxicity against Ephestiakuehniella. Biol. Control 37. 243–246.
Travers, R. S., Martin, P. A. W., and Reichelderfer, C. F. (1987) Selective process for efficient isolation of soil Bacillus species. App. Environ. Microbiol. 53, 1263–1266.
Lee, I. H., Je, Y. H., Chang, J. H., et al. (2001) Isolation and characterization of a Bacillus thuringiensis ssp. kurstaki strain toxic to Spodoptera exigua and Culex pipiens. Current Microbiol. 43, 284–287.
Ibarra, J. E. and Federici, B. A. (1986) Isolation of a relatively nontoxic 65-kilodalton protein inclusion from the parasporal body of Bacillus thuringiensis subsp. israelensis. J. Bacteriol. 165, 527–533.
Luo, K., Banks, D., and Adang, M. J. (1999) Toxicity, binding, and permeability analyses of four Bacillus thuringiensis Cry 1 d-endotoxins using brush border membrane vesicles of Spodoptera exigua and Spodoptera frugiperda. Appl. Environ. Microbiol. 65, 457–464.
Ruiz, L. M., Segura, C., Trujillo, J., and Orduz, S. (2004) In vivo binding of the Cry11Bb toxin of Bacillus thuringiensis subsp. medellin to the midgut of mosquito larvae (Diptera: Culicidae). Mem. Inst. Oswaldo Cruz. 99, 73–79.
Zouari, N. and Jaoua, S. (1997) Purification and immunological characterization of particular delta-endotoxins from strains of Bacillus thuringiensis. Biotechnol. Lett. 19, 825–829.
Lightwood, D. J., Ellar, D. J., and Jarrett, P. (2000) Role of proteolysis in determining potency of Bacillus thuringiensis Cry 1Ac delta-endotoxin. Appl. Environ. Microbiol. 66, 5174–5181.
Miranda R., Fermando Z. Z. and Bravo A. (2001) Processing of Cry 1Ab d-endotoxin from Bacillus thuringiensis by Manduca sexta and spodoptera frugiperda midgut proteases: role in protoxin activation. Insect Biochem. Mol. Biol. 31, 115–1163.
Levy, S. M., Falleiros, A. M. F., Moscardi, F., Gregorio, E. A., and Toledo, L. A. (2004) Morphological study of the hindgut in larvae of Anticarsia gemmatalis Hübner (Lepidoptera: Noctuidae). Neutropical Entomol. 33, 427–431.
Anderson, E. and Harvey, W. R. (1966) Active transport by the Cecropia midgut: II. Fine structure of the midgut epithelium. J. Cell. Biol. 31, 107–134.
Smith, D. S., Compher, K., Janners, M., Lipton, C., and Wittle, L. W. (1969) Cellular organization and ferritin uptake in the mid-gut epithelium of a moth Ephestia kuehniella. J. Morphol. 127, 41–72.
Jimenez, D. R. and Gilliam, M. (1990) Ultrastructure of the ventriculus of the hony bee, Apis mellifera (L.): cytochemical localization of acid phosphatase, alkaline phosphatase, and non-specific esterase. Cell Tissue res. 261, 431–443.
Terra, W. R. and Ferreira, C. (1994) Insect digestive enzymes: properties, compartementalization and function. Comp. Biochem. Physiol. 109B, 1–62.
Cristofoletti, P. T., Ribero, A. F. and Terra, W. R. (2001) Apocrine secretion of amylase and exocytosis of trypsin along the midgut of Tenebrio molitor larvae. J. Insect Physiol. 47, 143–155.
Segura, C. (2001) Study on the mode of action of Bacillus thuringiensis subsp medellin toxins. PhD Thesis, University of Antioquia, Medellin Colombia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rouis, S., Chakroun, M., Saadaoui, I. et al. Proteolysis, histopathological effects, and immunohistopathological localization of δ-endotoxins of Bacillus thuringiensis subsp. kurstaki in the midgut of lepidopteran olive tree pathogenic insect Prays oleae . Mol Biotechnol 35, 141–148 (2007). https://doi.org/10.1007/BF02686109
Issue Date:
DOI: https://doi.org/10.1007/BF02686109