Abstract
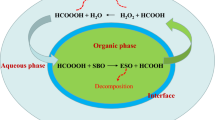
The kinetics of oxirane ring cleavage in epoxidized soybean oil have been studied using glacial acetic acid at 60, 70, 80 and 90°C. It was shown that the reaction can be successfully modelled as first order with respect to the epoxide concentration and second order with respect to acetic acid. The reaction velocity constant at 70°C was found to be 2 × 10−3 1−3 hr−1 mol−2, the frequency factor, A, = 2.321 × 107 hr−1 and the energy of activation, Ea = 15.84 k cal mol−1. The effects of the concentration of acetic acid and the temperature on the net yield of epoxides by in situ epoxidation were also studied on the basis of the predicted kinetic parameters of the reaction system.
Similar content being viewed by others
References
Rusling, J.F., G.R. Riser, M.E. Snook and W.E. Scott,J. Am. Oil Chem. Soc. 45:760 (1968).
Synthetic Organic Chemicals, U.S. Production and Sales (1963-1982), U.S. International Trade Commission, U.S. Government Printing Office, Washington, D.C.
Carlson, K.D., and S.P. Chang,J. Am. Oil Chem. Soc. 62:934 (1985).
Zaher, F.A., S.M. El-Shami and M.H. El-Mallah,Seifen Ole Fette Wachse 111:605 (1985).
Zaher, F.A., M.H. El-Mallah and M.M. El-Hefnawy, Fett Wissen-chaft Technologie, in press (1988).
Roswsky, A., inHeterocyclic Compounds with Three and Four Membered Rings, edited by A. Weissberger, Wiley Interscience, New York, 1964, pp. 181–459.
Swern, D.,J. Am. Oil Chem. Soc. 47:424 (1970).
Szakacs, S., S. Gobols and F. Nagy,J. Chem. Soc. Perkins Trans. II, 417–420 (1983).
Isaacs, N.S.,Tetrahedron Lett. 4549 (1965).
Isaacs, N.S., and K. Neelakantan,Can. J. Chem. 46:1043 (1968).
Schmitt, W.,Z. Phys. Chem. (Frankfurt) 59:217 (1968).
Frost, A.A., and R.G. Pearson,Kinetics and Mechanism, 2nd edn, John Wiley & Sons, Inc., New York, 1961.
Chalabiev, Ch.A., M.M. Guseinov and O.A. Sadygor,C.A. 93:47545S (1980).
Author information
Authors and Affiliations
About this article
Cite this article
Zaher, F.A., El-Mallah, M.H. & El-Hefnawy, M.M. Kinetics of oxirane cleavage in epoxidized soybean oil. J Am Oil Chem Soc 66, 698–700 (1989). https://doi.org/10.1007/BF02669955
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02669955