Abstract
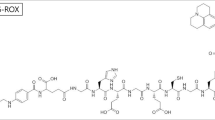
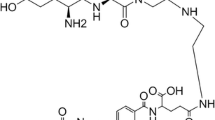
The need to develop target-specific MRI contrast agents to aid in disease characterization remains highly essential. In this study, we present a generation four polyamidoamine (PAMAM) folate-dendrimer that specifically targets the high affinity folate receptor (hFR) overexpressed on more than 80% of ovarian tumors. In vitro, mouse erythroleukemia cells expressing the hFR bind the radiolabeled folate-dendrimer chelate resulting in over 2700% increase in binding compared with untreated cells. The binding was inhibited by free folic acid to levels observed on folate-receptor-negative cells. In vivo, ovarian tumor xenografts resulted in a 33% contrast enhancement, following the folate-dendrimer chelate administration, that was significantly different compared with results obtained with a non-specific, extracellular fluid space agent, Gd-HP-DO3A. In addition, this contrast enhancement was absent in saline-treated animals, folate-receptor-negative tumors, and was inhibited by free folic acid. Results suggest that a macromolecular, dendrimeric MRI agent with high molecular relaxivities (1646 mM−1 s−1) can be used in specifically targeting the hFR on tumor cells and ovarian tumors.
Similar content being viewed by others
References
Brooks SE. Preoperative evaluation of patients with suspected ovarian cancer. Gynecol Oncol 1994;55:S80 -90.
Low RN. Saleh F. Song SYT, et al. Treated ovarian cancer: comparison of MR imaging with serum CA-125 level and physical examination — a longitudinal study. Radiology 1999;211:519–28.
Brasch RC. Rationale and applications for macromolecular Gd-based contrast agents. Magn Reson Med 1994;22:282–7.
Lauffer RB. Paramagnetic metal complexes as water proton relaxation agents for NMR imaging: theory and design. Chem Rev 1987;87:901–27.
Unger EC, Totty WG. Neufeld DM, et al. Magnetic resonance imaging using gadolinium labeled monoclonal antibody. Invest Radiol 1985;20:693–700.
Gohr-Rosenthal S, Schmit-Willich H, Ebert W, et al. The demonstration of human tumors on nude mice using gadolinium-labeled monoclonal antibodies for magnetic resonance imaging. Invest Radiol 1993;28:789–95.
Mendonca-Dias MH, Lauterbur PC. Ferromagnetic particles as contrast agents for magnetic resonance imaging of the liver and spleen. Magn Reson Med 1986;3:328–30.
Renshaw PF. Owen CS, McLaughlin AC, et al. Ferromagnetic contrast agents: a new approach. Magn Reson Med 1986;3(2):217–25.
Cerdan S. Lotscher HR, Kunnecke B. et al. Monoclonal antibody-coated magnetite particles as contrast agents in magnetic resonance imaging of tumors. Magn Reson Med 1989;12:151–63.
Weissleder R, Lee AS, Fischman AJ, et al. Polyclonal human immunoglobulin G labeled with polymeric iron oxide: antibody MR imaging. Radiology 1991;181:245–9.
Weissleder R, Lee AS, Khaw BA, et al. Antimyosin-labeled monocrystalline iron oxide allows detection of myoeardial infarct: MR antibody imaging. Radiology 1992;182:381–5.
Reimer P, Weissleder R, Shen T, et al. Pancreatic receptors: initial feasibility studies with a targeted contrast agent for MR imaging. Radiology 1994;193:527–31.
Reimer P, Weissleder R, Lee AS, et ai. Receptor imaging: application to MR imaging of liver cancer. Radiology 1990;177:729–34.
Kresse M, Wagner S, Pfefferer D, et al. Targeting of ultrasmall superparamagnetic iron oxide (USPIO) particles to tumor cells in vivo by using transferrin receptor pathways. Magn Reson Med 1988;40:236–42.
Sipkins DA, Cheresh DA, Kazemi MR. et al. Detection of tumor angiogenesis in vivo by αvβ3targeted magnetic resonance imaging. Nature Med 1998;4:623–6.
Sipkins DA, Gijbels K, Tropper FD, et al. ICAM-1 expression in autoimmune encephalitis visualized using magnetic resonance imaging. J Neuroimmunol 2000;104:1–9.
Wiener EC, Brechbiel MW, Brothers H, et al. Dendrimer-based metal chelates: a new class of magnetic resonance imaging contrast agents. Magn Reson Med 1994;31:1–8.
Roberts JC, Adams YE, Tomalia D, et al. Using starburst dendrimers as linker molecules to radiolabel antibodies. Biocon-jugate Chem 1990;l(5):305–8.
Yang W, Barth RF, Adams D. et al. Intratumoral delivery of boronated epidermal growth factor for neutron capture therapy of brain tumors. Cancer Res 1997;57:4333–9.
Wiener EC, Konda S. Shadron A. et al. Targeting dendrimer-chelates to tumors and tumor cells expressing the high-affinity folate receptor. Invest Radiol 1997;32:748–54.
Konda SD, Aref M, Brechbiel M. et al. The development of a tumor targeting magnetic resonance contrast agent using the high affinity folate receptor: work in progress. Invest Radiol 2000;35:50–7.
Zuckier LS, Rodriguez LB, Scharff MD. Immunologie and pharmacologie concepts of monoclonal antibodies. Semin Nucl Med 1989;28:166–86.
Leamon CP, Low PS. Delivery of macromolecules into living cells: a method that exploits folate receptor endocytosis. Proc Natl Acad Sci 1991;88:5572–6.
Leamon CP, Low PS. Cytotoxicity of mormordin-folate conjugates in cultured human cells. J Biol Chem 1992;267:24966–71.
Leamon CP, Pastan I, Low PS. Cytotoxicity of folate-psuedomonas exotoxin conjugates toward tumor cells. J Biol Chem 1993;268:24847–54.
Ross JF, Chaudhuri PK, Ratnam M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Cancer 1994;73:2432–43.
Weitman SD, Weinberg AG, Coney LR, et al. Cellular localization of the folate receptor: potential role in drug toxicity and folate homeostasis. Cancer Res 1992;52:6708–11.
Campbell IG, Jones TA, Foulkes WD, et al. Folate-binding protein is a marker for ovarian cancer. Cancer Res 1991;51:5329–38.
Miotti S, Canevari S, Menard S, et al. Characterization of human ovarian carcinoma-associated antigens by novel monoclonal antibodies with tumor-restricted specificity. Int J Cancer 1987;39:297–303.
Veggian R, Fasolato S. Menard S, et al. Immunohistochemical reactivity of a monoclonal antibody prepared against human ovarian carcinoma on normal and pathological female genital tissues. Tumori 1989;75:510–3.
Coney LR, Tomassetti A, Carayannopoulos L, et al. Cloning of a tumor-associated antigen: MOv 18 and MOv l9 antibodies recognize a folate-binding protein. Cancer Res 1991;51:6125–32.
Weitman SD, Weinberg AG, Coney LR, et al. Cellular localization of the folate receptor: potential role in drug toxicity and folate homeostasis. Cancer Res 1992;52:6708–11.
Garin-Chesa P, Campbell I, Saigo PE, et al. Trophoblast and ovarian cancer antigen LK26. Am J Pathol 1993; 142:557–67.
Weitman SD, Lark RH, Coney LR, et al. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res 1992;52:3396–401.
Weitman SD, Frazier KM, Kamen BA, et al. The folate receptor in central nervous system malignancies of childhood. J Neurooncol 1994;2l: 107–12.
Gor’Kov PL, Harris AB, Lauterbur PC. Immobilization system for rat brain imaging. Proc ISMRM 1998;3:2054.
Jain JK. Transport of molecules in the tumor interstitium: a review. Cancer Res 1987;47:3039–51.
Rijnboutt S, Jansen G, Posthuma G, et al. Endocytosis of GPI-linked membrane folate-receptor-[alpha]. J Cell Biol 1996;132:35–47.
Luhrs CA, Slomiany BL. A human membrane-associated folate binding protein is anchored by a glycosyl-phospatidylinositol tail. J Biol Chem 1989;264:21446–9.
Kane MA, Elwood PC, Portilla RM, et al. The interrelationship of the soluble and membrane-associated folate-binding proteins in human KB cells. J Biol Chem 1986;261:15625–31.
Antony AC. The biological chemistry of folate receptors. Blood 1992;79:2807–20.
Toffolli G, Cernigo C, Russo A, et al. Over expression of folate binding protein in ovarian cancers. Int J Cancer 1997;74:193–8.
Toffoli G, Russo A. Gallo A, et al. Expression of folate binding protein as a prognostic factor for response to platinum-containing chemotherapy and survival in human ovarian cancer. Int J Cancer 1998;79:121–6.
Corona G, Giannini F. Fabris M, et al. Role of folate receptor and reduced folate carrier in the transport of 5-mcthyltetrahydrofolic acid in human ovarian carcinoma cells. Int J Cancer 1998;75:125–33.
Nunn AD, Linder KE, Tweedle MF. Can receptors be imaged with MRI agents. Q J Nucl Med 1997;41:155–62.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Konda, S.D., Aref, M., Wang, S. et al. Specific targeting of folate–dendrimer MRI contrast agents to the high affinity folate receptor expressed in ovarian tumor xenografts. MAGMA 12, 104–113 (2001). https://doi.org/10.1007/BF02668091
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02668091