Abstract
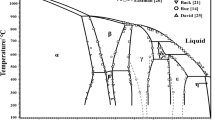
The Zn-Se phase diagram is reanalyzed with an associated solution model for the liquid, new Se-rich liquidus points, and a new Gibbs energy of formation for ZnSe(s). After the liquid model parameters are fixed by a best fit to the liquidus points, the entire liquidus is generated as well as the partial pressures of Zn and of Se2 along the ZnSe(s) three-phase curve, isotherms for the enthalpy of mixing, and the isotherm for the activity coefficients at the 1799 K melting point of ZnSe(s).
Similar content being viewed by others
Cited References
W.J. Wosten and M.G. Geers,J. Phys..Chem., 66, 1252 (1962). ;
R. Hultgren, R.L. Orr, P.O. Anderson, and K.K. Kelley,Selected Thermodynamic Properties of Metals and Alloys, John Wiley and Sons (1963).
E.I. Boev, L. A. Benderskii, and G. A. Milkov,Zh. Fiz. Khitn., 43, 1393 (1964).
L.A. Sysoev, E.K. Raiskin, and V.R. Gurev,Inorganic Mater., 3, 342 (1967).
M. Rubenstein,J. Cryst. Growth, 3/4, 309 (1968).
L.R. Shiozawa and J.M. Jost, U.S. Govt. Rept. AD-660, p 874 (1968).
R. Hultgren, P.D. Desai, D.T. Hawkins, M. Gleiser, K.K. Kelley, and D.D. Wagman,Selected Values of the Thermodynamic Properties of the Elements, American Society for Metals, Metals Park, OH (1973).
L. Ozawa and H.N. Hersh,J. Electrochem. Soc., 120(7), 938 (1973).
L.P. Kuzhelev, I.A. Mironov, V.N. Pavlova, and I.M. Stroganova,Russ. J. Phys. Chem. 48(2), 287 (1974).
K.C. Mills,Thermodynamic Data for Inorganic Sulphides, Selenides, and Tellurides, Butterworth, London (1974).
G. Newns; quoted inThermodynamic Data for Inorganic Sulphides, Selenides, and Tellurides, Butterworth, London (1974)[74Mil].
V.M. Lakeenov and O. V. Pelevin,Russ. J. Phys. Chem., 57(3), 453 (1977).
I. Kikuma and M. Furukoshi,J. Cryst. Growth, 50, 654 (1980).
M. Aoki, M. Washiyama, H. Nakamura, and K. Sakamoto,Jpn. J.Appl.Phys., 21, 11 (1982).
R.F. Brebrick, C.H. Su, and P.K. Liao,Semiconductors and Semimetals, Vol. 19, R.K. Willardson and A.C. Beer, Ed., Academic Press, NY (1983).
A. Marbeuf, M. Feerah, E. Janik, and A. Huertel,J. Cryst. Growth, 72, 126 (1985).
R.C. Sharma and Y.A. Chang,J. Cryst. Growth, 88, 193 (1988).
A. Nasar and M. Shamsuddin,Z. Metallkd., 81, 244 (1990).
H. Unuma, M. Higuchi, Y. Yamakawa, K. Kodaira, Y. Okano, K. Hoshikawa, T. Fukuda, and T. Koyama,Jpn. J. Appl. Phys., 31, Pt.2, L383 (1992).
R.F. Brebrick and H. Liu,High Temp, and Mater. Sci., 35, 215 (1996).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brebrick, R.F., Liu, H. Analysis of the Zn-Se system. JPE 17, 495–501 (1996). https://doi.org/10.1007/BF02665996
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02665996