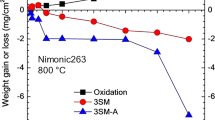
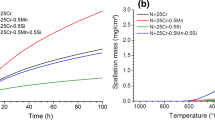
Abstract
According to the “oxide ion” theory, sulfidation attack does not occur until oxide ions present in the fused Na2SO4 melt react with the normally protective oxide scale. It has already been shown that chromia reacts with and decreases the oxide ion content of sodium sulfate and inhibits sulfidation attack. Based upon the results reported herein, the reduction of the oxide ion content of sodium sulfate is a necessary but not sufficient condition for sulfidation inhibition. It is shown that the oxides of molybdenum as well as vanadium react with and decrease the oxide ion content of Na2SO4. It is shown that the addition of either Mo or V to nickel imparts sulfidation resistance. However, it is also shown that whereas the addition of Cr2O3 to Na2SO4-coated nickel-base superalloys prevents or inhibits sulfidation attack, no beneficial effects are noted when either MoO3 or V2O5 are codeposited with Na2SO4 onto nickel-base superalloy substrates. The reactions between V2O5 with metal oxides were also studied. V2O5 readily fluxes Al2O3 and slowly reacts with Na2SO4. The relationship between accelerated oxidation, oxide ion content of a fused melt and the fluxing of the normally protective oxide scale by liquid metal oxides is discussed.
Similar content being viewed by others
References
M. A. DeCrescente and N. S. Bornstein:Corrosion, 1968, vol. 24, pp. 127–33.
E. L. Simins, G. V. Browning, and H. A. Liebhefsky:Corrosion, 1955, vol. 11, no. 12, p. 505.
G. J. Danek, Jr.:Nav. Eng. J. Dec. 1965, p. 859.
A. U. Seybolt:Trans. TMS-AIME, 1968, vol. 242, pp. 1955–61.
J. M. Quets and W. H. Dresher:J. Mater., 1969, vol. 4, pp. 583–99.
N. S. Bornstein and M. A. DeCrescente:Trans. TMS-AIME, 1969, vol. 245, pp. 1947–52.
N. S. Bornstein and M. A. DeCrescente: Investigation of Sulfidation Mechanism in Nickel Base Superalloys. Final Report conducted for U.S. Naval Ship Research and Development Laboratory, Contract N00600-68-C-0639 (April 1969) Annapolis, Md.
N. S. Bornstein and M. A. DeCrescente:Met. Trans., 1971, vol. 2, pp. 1971–83.
J. A. Goebel and F. S. Pettit:Met. Trans., 1970, vol. 1, pp. 1943–54.
N. S. Bornstein, M. A. DeCrescente, and H. A. Roth:Corrosion, 1972, vol. 28, no. 7, pp. 264–68.
The Mechanism of Corrosion by Fuel Impurities, Butterworths, 1963.
G. Danner and M. Rey:Electrochem. Acta, 1961, vol. V, pp. 274–87.
C. H. Liu:J. Phys. Chem., 1962, vol. 66, no. 1, pp. 164–66.
U. S. Bureau of Mines Report of Investigation 7371, April 1970.
N. S. Bornstein, M. A. DeCrescente, and T. Sturiale: Proc. of the Air Force Materials Lab Fiftieth Tech. Conf. on Corrosion of Military and Aerospace Equipment, Tech. Report AFML-TR-67-329, Nove. 1967, pp. 1476–95.
V. V. Illanonov, R. P. Ozerov, and E. Kildisheva:Zh. Neorg. Khim., 1957, vol. 2, pp 884.
J. A. Goebel, F. S. Pettit, and G. W. Goward: Pratt & Whitney Aircraft Division of United Aircraft Corporation, East Hartford, Connecticut, Personal Communication.
H. Morrow, III and E. Kalns: Climax Molybdenum Co. Report RP 57-68-01, May, 1971.
References
Na2SO4 W. J. Cooper and D. A. Scarpullo: Final Report, SC-RR-64-67, Calleny Chemical Co. to Sandia Corp., Jan. 1964.
Na2MoO4 W. J. Cooper and D. A. Scarpullo: Final Report, SC-RR-64-67, Calleny Chemical Co. to Sandia Corp., Jan. 1964.
Na2CrO4 W. J. Cooper and D. A. Scarpullo: Final Report, SC-RR-64-67, Calleny Chemical Co. to Sandia Corp., Jan. 1964.
Na2OThermochemistry for Steelmaking, Addison-Wesley, 1960.
Na2SThermochemistry for Steelmaking, Addison-Wesley, 1960.
Cr2O3 Thermochemistry for Steelmaking, Addison-Wesley, 1960.
SO2 Janaf Thermomechamical Tables. The Dow Chemical Co., Midland, Michigan.
NaAlO2 J. P. Coughlin:J. Am. Chem. Soc., 1957, vol. 79, p. 2397.
Al2(SO4)3 High Temperature Properties and Decomposition of Inorganic Salts—Part I, U. S. Dept. of Commerce, National Bureau of Standards, NSRDS-NBS-7, 1966.
V2O5 A. D. Mah: Report of Investigations 6727, Bureau of Mines, U. S. Dept. of Interior, 1966.
NaVO3 A. D. Mah: Report of Investigations 6727, Bureau of Mines, U. S. Dept. of Interior, 1966.
Na2CrO4 L. A. Zharkova and Y. Gerasimov:Zh. Fiz. Khim., 1961, vol. 35, pp. 2291–96.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bornstein, N.S., DeCrescente, M.A. & Roth, H.A. The relationship between relative oxide ion content of Na2SO4, the presence of liquid metal oxides and sulfidation attack. Metall Trans 4, 1799–1810 (1973). https://doi.org/10.1007/BF02665406
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02665406