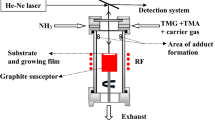
Abstract
Decomposition of o-CH3C6H4AsD2 in the gas phase at 600–1000°C produces toluenes with 0-3 D atoms in the methyl group. It is shown that this cannot be accounted for by conventional mechanisms involving initial As-C bond cleavage or reductive elimination, but rather that the first step is As-D bond cleavage and this is followed by reductive elimination of o-CH3C6H4D or H atom transfer to give o-HDAsC6H4CH2· which abstracts D from an intact o-tolylarsine to give o-CH2DC6H4AsHD. Repetition of these steps can lead to multiple D incorporation. The free energies of activation for reductive elimination or multiple D incorporation are found to be very similar. Theoretical studies on the decomposition oftBuAsH2 show that the first step for decomposition can be As-H bond cleavage to givetBuAsH· or loss of H2 totBuAs.tBuAsH· decomposes totBu· which abstracts H· fromtBuAsH2 to give 2-methylpropane or by β-H abstraction to give 2-methylpropene.tBuAs, on the other hand only gives 2-methylpropene,via a β-H abstraction mechanism. Measured effects of total reactor pressure on product distribution are modeled qualitatively. Hex-5-enylarsine also decomposesvia initial As-H bond cleavage followed by reductive elimination of 1-hexene. However, it reacts in the liquid or solution phase with Me3Ga to give the adduct. [Me3Ga.AsH2hex]. On heating, this loses methane to give first [Me2Ga.AsHhex]3 then [MeGa.Ashex]n. Finally, GaAs is produced with the formation of methane and methylenecyclopentane. The last product indicates a free radical mechanism involving cleavage of the As-hex bond for the last step. In the gas phase at 600°C, GaAs is again formed but the major C6 product is 1-hexene. This is interpreted as meaning that the adduct, [Me3Ga.AsH2hex] is not formed in the gas phase under growth conditions.
Similar content being viewed by others
References
P. Zanella, G. Rossetto, N. Brianese, F. Ossola, M. Porchia and J.O. Williams,Chem. Mater. 3, 225 (1991).
G. Haake, S.P. Watkins and H. Burkhard,J. Cryst. Growth 107, 342 (1991).
R.D. Hoare, O.F.Z. Khan, J.O. Williams, D.M. Frigo,D.C. Bradley, H. Chudzynska, P. Jacobs, A.C. Jones andS.A. Rushworth,Chemtronics 4, 78, (1989).
R.M. Lum and J.K. Klingert,J. Cryst. Growth 107, 290(1991).
See Reactions of Organometallic Compounds with Surfaces, eds D.J. Cole-Hamilton and J.O. Williams, (New York: Plenum, 1989).
P.-W. Lee, T.R. Omstead, D.R. McKenna and K.F. Jensen,J. Cryst.Growth, 93, 134 (1988).
C.A. Larsen, N.I. Buchan, S.H. Li and G.B. Stringfellow,J. Cryst. Growth 94, 663 (1989).
R.H. Marking, W.L. Gladfelter and K.F. Jenson,Chem. Mater. 2, 499(1990).
D.F. Foster, C. Glidewell and D.J. Cole-Hamilton,Appl. Phys. Lett. 63, 57 (1993).
D.F. Foster, C. Glidewell and D.J. Cole-Hamilton,Appl. Phys. Lett. 63, 214(1993).
M.C. Ball and A.H. Norbury,Physical Data for Inorganic Chemists (London: Longman, 1974).
J.J.P. Stewart,J. Comp. Chem. 10, 209, (1989).
J.J.P. Stewart,J. Comp. Chem. 12, 320 (1991).
C.A. Larsen, N.I. Buchan and G.B. Stringfellow,Appl. Phys. Lett. 52, 480 (1988).
J.J.P. Stewart,J. Computer-Aided Molecular Design4, 1 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Foster, D.F., Glidewell, C. & Cole-Hamilton, D.J. Probing the mechanisms of growth of gallium arsenide by metalorganic vapor phase epitaxy using experimental and theoretical studies of designed precursors. J. Electron. Mater. 23, 69–74 (1994). https://doi.org/10.1007/BF02655248
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02655248