Abstract
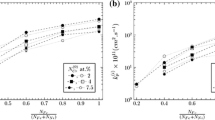
The internal oxidation of iron alloys containing between 0.069 and 0.274 wt pct aluminum was investigated in the temperature range from 1223 to 1373 K for the purpose of determining the diffusion coefficients in γ-iron as well as in the internal oxidation layer. A parabolic rate law is obeyed in the internal oxidation of the present alloys. The rate constant for penetration of the oxidation front, the oxide formed, and the concentration of aluminum in the oxidation layer were determined. Pronounced enrichment of aluminum in the oxidation layer was observed, resulting from the counterdiffusion of aluminum. The oxygen concentration at the specimen surface was determined by combining the thermodynamic data on the dissociation of FeO and the solution of oxygen in y-iron. The diffusion coefficient of oxygen in the internal oxidation layer,D 10o , was evaluated on the basis of the rate equation for internal oxidation.D 10o increases at a given temperature as the volume fraction of oxide,f 10, in the oxidation layer increases. The diffusion coefficient of oxygen in γ-iron,D o, was determined by extrapolation ofD 10o = 0.D o may be expressed as
D o is close to the diffusion coefficients of carbon and nitrogen in γ-iron.
Similar content being viewed by others
References
J. L. Meijering:Advances in Materials Sci., 1971, vol. 5, pp. 1–81.
J. H. Swisher:Oxidation of Metals and Alloys, ASM, Metals Park, OH, 1971, pp. 235–67.
R. A. Rapp:Corrosion, 1965, vol. 21, pp. 382–401.
J.E. Verfurth and R.A. Rapp:Trans. TMS-AIME, 1964, vol. 230, pp. 1310–13.
S. Goto, K. Nomaki, and S. Koda:J. Jpn. Inst. Met., 1967, vol. 31, pp. 600–06.
S. Goto and S. Koda:J. Jpn. Inst. Met., 1970, vol. 34, pp. 319–26.
H. Schenck, E. Schmidtmann, and H. Müller:Arch. Eisenhiittenw., 1960, vol. 31, pp. 121–28.
K. Bohnenkamp and H.J. Engell:Arch.Eisenhüttenw., 1964, vol. 35, pp. 1011–18.
M.T. Hepworth, R.P. Smith, and E.T. Turkdogan:Trans. TMS-AIME, 1966, vol. 236, pp. 1278–83.
J.H. Swisher and E.T. Turkdogan:Trans. TMS-AIME, 1967, vol. 239, pp. 426–31.
J. Takada, K. Kashiwagi, and M. Adachi:J. Materials Sci., 1984, vol. 19, pp. 3451–58.
J.A. Kitchener, J. O’M. Bockris, Molly Gleiser, and J. W. Evans:Acta Metall., 1953, vol. 1, pp. 93–101.
F.R. Rhines, W. A. Johnson, and W. A. Anderson:Trans. TMS-AIME, 1942, vol. 147, pp. 205–21.
C. Wagner:Z. Elektrochem., 1959, vol. 63, pp. 772–82.
F. Maak:Z. Metallic., 1961, vol. 52, pp. 545–46.
R.A. Rapp:Acta Metall., 1961, vol. 9, pp. 730–41.
G. Böhm and M. Kahlweit:Acta Metall., 1964, vol. 12, pp. 641–48.
J. Pötschke, P.M. Mathew, and M.G. Frohberg:Z. Metallic., 1970, vol. 61, pp. 152–55.
T. Igarashi and M. Osada:J. Jpn. Inst. Met., 1980, vol. 44, pp. 1224–29.
J.H. Swisher:Trans. TMS-AIME, 1968, vol. 242, pp. 1035–38.
O. Kubaschewski, E.L1. Evans, and C.B. Alcock:Metallurgical Thermochemistry, 4th ed., Pergamon Press, London, 1967, p. 423.
R. P. Smith:Trans. TMS-AIME, 1964, vol. 230, pp. 476–80.
P. Grieveson and E. T. Turkdogan:Trans. TMS-AIME, 1964, vol. 230, pp. 407–14.
Author information
Authors and Affiliations
Additional information
MASAO ADACHI, formerly Professor
SADAHIRO YAMAMOTO, formerly Graduate Student of Kyoto University
Rights and permissions
About this article
Cite this article
Takada, J., Yamamoto, S., Kikuchi, S. et al. Determination of diffusion coefficient of oxygen in γ-iron from measurements of internal oxidation in Fe-Al alloys. Metall Trans A 17, 221–229 (1986). https://doi.org/10.1007/BF02643898
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02643898