Abstract
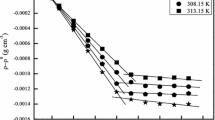
Viscosity measurements of calcium soaps show that two kinds of micelles are formed in aqueous methanol mixtures. The change in the nature of micelles from hydrophilic oleomicelles to lipophilic hydromicelles occurs in solvents containing 40–50% (v/v) of methanol. The equations of Vand and Moulik are applicable only above the critical micelle concentration of the soaps. The parameters of the equations have been evaluated. These may be used to calculate the viscosity of soap solutions in the concentration range in which the equations hold good.
Similar content being viewed by others
References
Varma, R.P., and P. Bahadur, Cellul. Chem. Technol. 8:27 (1974).
Schulman, J.H., and D.P. Riley, J. Colloid Sci. 3:383 (1948).
Vand, V., J. Phys. Colloid. Chem. 52:277 (1948).
Moulik, S.P., J. Phys. Chem. 72:4682 (1968).
“International Encyclopedia of Physical Chemistry and Chemical Physics,” Topic 16, Vol. 3, Pergamon Press, Oxford, England, 1965, p. 4f.
Moulik, S.P., J. Indian Chem. Soc. 49:483 (1972).
Author information
Authors and Affiliations
About this article
Cite this article
Varma, R.P., Bahadur, P. Studies on viscosity of calcium soaps in aqueous methanol. J Am Oil Chem Soc 51, 545–547 (1974). https://doi.org/10.1007/BF02636026
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02636026