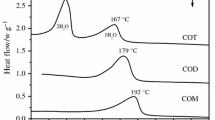
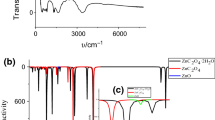
Abstract
Geometries and energies of isolated CaC2O4·H2O, CaC2O4, CaCO3, CaO, H2O, CO and CO2 were determined at the ab initio level using effective core potential valence basis sets of doublezeta quality, supplemented with polarization functions. The effects of electron correlation were taken into account at the second order Møller-Plesset level of theory. For CaC2O4·H2O, the correlation for the basis set superposition error was also included. Common routines were employed to evaluate entropies, heat capacities, as well as enthalpies and free enthalpies of formation of all entities. The enthalphies and free enthalpies of consecutive dehydration of CaC2O4·H2O, decarbonylation of CaC2O4 and decomposition of CaCO3 towards CaO and CO2 were determined on the basis of avialable data from the literature or those predicted thoretically. Assuming that upon all the above mentioned processes the system maintains equilibrium, the fractions reacted, enthalpy changes and differential dependencies of thesevs. temperature were derived and compared with experimental thermoanalytical data.
Zusammenfassung
Geometrie und Energien von isoliertem CaC2O4·H2O, CaC2O4, CaCO3, CaO, H2O, CO und CO2 wurden auf dem ab initio Level unter Anwendung von Kernpotential-Valenz-Basissets, ergänzt durch Polarisationsfunktionen bestimmt. Die Effekte der Elektronenkorrelation wurden auf dem Moller-Plesset level zweiten Grades berücksichtigt. Für CaC2O4·H2O wurde auch die Korrelation für den Basissetüberlagerungsfehler inbegriffen. Zur Ableitung der Entropien, Wärmekapazitäten als auch Enthalpien und freien Bildungsenthalpien aller Gebilde wurden die üblichen Routinen verwendet. Die Enthalpien und freien Enthalpien der konsekutiven Dehydratation von CaC2O4·H2O, der Dekarbonylierung von CaC2O4 und der Zersetzung von CaCO3 zu CaO und CO2 wurde auf der Grundlage der zugänglichen Literaturangaben bestimmt. Unter der Annahme, daß das System in allen oben erwähnten, Prozessen im Gleichgewicht bleibt, wurden die Enthalpieänderungen und differentialen Abhängigkeiten derselben von der Temperature abgeleitet und mit den experimentellen thermoanalytischen Angaben verglichen.
Similar content being viewed by others
References
Atlas of Thermoanalytical Curves, G. Liptay (Ed.), Akadémiai Kiadó, Budapest 1973.
J. Paulik and F. Paulik, Comprehensive Analytical Chemistry, Vol. 12, Part A Elsevier, Amsterdam 1981, p. 83.
M. R. Alvarez, J. J. Icaza, E. H. Bocanegra and M. J. Tello, Thermochim. Acta, 12 (1975) 117.
C. G. R. Nair and K. N. Ninan, Thermochim. Acta, 23 (1978) 161.
K.' N. Ninan and C. G. R. Nair, Thermochim. Acta, 37 (1980) 161.
J. Blazejowski, J. Szychlinski and K. Windorpska, Thermochim. Acta, 46 (1981) 147.
G. Beech, J. Chem. Soc. A, (1969) 1903.
M. S. Subramania, R. N. Singh and H. D. Sharma, J. Inorg. Nucl. Chem., 31 (1969) 3789.
S. Gurrieri, G. Siracusa and R. Cali, J. Thermal Anal., 6 (1974) 293.
J. Baker, J. Comput. Chem., 7 (1986) 385.
W. J. Stevens, H. Basch and M. Krauss, J. Chem. Phys., 81 (1984) 6026.
M. Krauss, W. J. Stevens, H. Basch and P. G. Jasien, Can. J. Chem., 70, (1992) 612.
W. J. Hehre, L. Radom, P. v. R. Schleyer and J. A. Pople, Ab initio Molecular Orbital Theory, Wiley, New York 1986.
M. Dupuis, D. Spangler and J. J. Wendoloski, National Resource for Computations in Chemistry Software Catalog; University of California: Berkeley, 1980 (Program QD 01); current version M. W. Schmidt, K. K. Baldridge, J. A. Boatz, J. H. Jensen, S. Koseki, M. S. Gordon, K. A. Nguyen, T. L. Windus and S. T. Elbert, QCPE Bull., 10 (1990) 52.
C. Møller and M. S. Plesset, Phys. Rev., 46 (1934) 618.
M. Gutowski, F. B. van Duijneveldt, G. Chalasinski and L. Piela, Mol. Phys., 61 (1987) 233.
J. A. Pople, B. T. Luke, M. J. Frish and J. S. Binkley, J. Phys. Chem., 89 (1985) 2198.
P. W. Atkins, Physical Chemistry, W. H. Freeman, New York, 3rd edn., 1986.
Handbook of Chemistry and Physics, D. R. Lide (Ed.), 73 rd edn. CRS Press, Boca Raton 1992–1993.
Gmelins Handbuch der Anorganischen Chemie, 8. Auflage, Teil B-Lieferung 3, Verlag Chemie, Weinheim/Bergstrasse, 1961.
K. P. Mishchenko and A. A. Ravdel, Kratki Spravochnik Fiziko Khimicheskikh Velichin, 5th edn., Khimiya, Leningrad 1967.
J. C. Bailar, H. J. Emeleus, R. Nyholm and A. F. Trotman-Dickenson (Eds.), Comprehensive Inorganic Chemistry, Pergamon Press Oxford 1975, chapters 10 and 13.
Handbook of Chemistry and Physics, 59th edn., CRS Press Boca Raton, 1978/1979.
J. Lubkowski and J. Blazejowski, J. Chem., Soc. Faraday Trans. 1, 82 (1986) 3069.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rak, J., Skurski, P., Gutowski, M. et al. Thermodynamics of the thermal decomposition of calcium oxalate monohydrate examined theoretically. Journal of Thermal Analysis 43, 239–246 (1995). https://doi.org/10.1007/BF02635991
Issue Date:
DOI: https://doi.org/10.1007/BF02635991