Summary
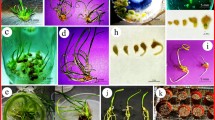
Haworthia comptoniana specimens were cultured to determine how benzyladenine (BA) level and in vitro selection for shoot and callus production affected regeneration capacity and plant phenotype. Leaf explants were cultured on Murashige and Skoog medium containing 0 to 10 mg·liter−1 of BA. The highest number of shoots was obtained with 0.5 mg·liter−1 of BA.H. comptoniana stock cultures (hc) maintained with 0.5 mg·liter−1 of BA produced clumps of small shoots interspersed with friable, white, tan, and green callus. A clump of very large shoots was isolated and designated cell line Rhc; it differed from the original hc culture in shoot size, the lack of callus growth, and higher water content. A line of green callus (designated Gc), a line of white callus (Wc), and a line of soft tan callus (Tc) were also isolated from hc. Optimal BA levels for shoot regeneration from lines Gc and Wc were 2 and 5 mg·liter−1, respectively. No normal shoots could be regenerated from Tc. The phenotypes of these cell lines remained stable for 24 subculture generations. The hc line that initially required BA for growth became hormone autotrophic whereas the other lines did not. Culturing using Gelrite and sealing vessels with parafilm promoted vitrification of the hc line. Culturing using GIBCO agar and unsealed vessels reduced vitrification. The ex-vitro greenhouse survival rates for hc and Rhc plantlets were 10 and 80%, respectively. The large size of the Rhc shoots apparently resulted in significantly higher survival rates under greenhouse conditions, but did not result in any phenotypic whole plant changes.
Similar content being viewed by others
References
Bayer, M. D. The newHaworthia handbook. National Botanic Gardens of South Africa; 1982:124.
Bottcher, I.; Zoglauer, K.; Goring, H. Induction and reversion of vitrification of plants culturedin vitro. Physiol. Plant 72:560–564; 1988.
Fox, J. E. Growth factor requirements and chromosome number in tobacco tissue cultures. Physiol. Plant 16:793–803; 1963.
Kaminek, M.; Lustinec, J. Reduced chlorophyll synthesis in cytokininautonomous strains of tobacco callus. Z. Pflanzenphysiol. 73:65–73; 1974.
Kaul, K.; Sabharwal, P. S. Morphogenetic studies onHaworthia: establishment of tissue culture and control of differentiation. Am. J. Bot. 59:377–385; 1972.
Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 15:473–497; 1962.
Ogihara, Y. Characterization of chromosomal changes in calluses induced by callus cloning and para-fluorophenylalanine (PFP) treatment and regenerates from them. Proc. 5th Intl. Cong. Plant Tissue & Cell Culture. Plant Tissue Culture. Tokyo, Japan: Japanese Association for Plant Tissue Culture; 1982:439–440.
Ogihara, Y.; Tsunewaki, K. Tissue culture inHaworthia. III. Occurrence of callus variants during subcultures and its mechanism. Jpn. J. Genet. 54:271–293; 1979.
Singh, B. D.; Harvey, B. L.; Kao, K. N., et al. Selection pressure in cell populations ofVicia hajastana cultured in vitro. Can. J. Genet. Cytol. 14:65–70; 1972.
Standifer, L. C.; O’Rourke, E. N.; Porche-Sorbet, R. Propagation ofHaworthia from floral scapes. Plant Propagator 30:4–6; 1984.
Steel, R. G. D.; Torrie, J. H. Observations. In: Steel, R. G. D.; Torrie, J. H., eds. Principles and procedures of statistics with special reference to the biological sciences. New York: McGraw-Hill; 1960:7–31.
Syono, K.; Furuya, T. Induction of auxin nonrequiring tobacco calluses and its reversal by treatments with auxin. Plant Cell Physiol. 15:7–17; 1974.
Wessels, D. C.; Groenewald, E. G.; Koeleman, A. Callus formation and subsequent shoot and root development from leaf tissue ofHaworthia planifolia cf. var.setulifera v. Poelln. Z. Pflanzenphysiol. Bd. 78:141–145; 1976.
Zimmerman, T. W.; Rogers, S. M. D.; Cobb, B. G. Controlling vitrification of petuniain vitro. In Vitro Plant Cell. Dev. Biol. 27:165–167; 1991.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dethier Rogers, S.M. Culture phenotype affects on regeneration capacity in the monocotHaworthia comptoniana . In Vitro Cell Dev Biol - Plant 29, 9–12 (1993). https://doi.org/10.1007/BF02632232
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02632232