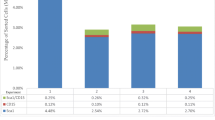
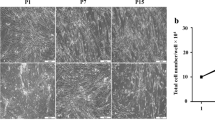
Summary
Cell cultures of primary mouse granulosa cells were transfected with a v-myc-containing plasmid, and the resulting stable cell lines were tested for their steroidogenic properties and physiologic status. Granulosa cells were obtained from 22-day-old NMRI mice injected with 8 IU pregnant mare serum gonadotropin i.p. 2 days earlier. In Passage 1 the cells were transfected with pSVv-myc using calcium phosphate precipitation or lipofectin. The 3β- and 17β-hydroxy steroid dehydrogenase activity was visualized in control cultures. The three cell lines obtained have been in culture for over 1 yr and have been subcultured for more than 90 passages. The cell line GRM01, with a doubling time of 37±3 h and a diploid modal chromosome number, produced progesterone, estradiol, as well as inhibinlike and activinlike material under basal conditions. A combination of follicle-stimulating hormone and luteinizing hormone was able to increase the secretion of progesterone. GRM01L, a fast growing clone of the GRM01 line with a doubling time of 10±1 h, retained only the capacity to produce activinlike material and transforming growth factor-β, and it was the only one with a tumorigenic capacity. Epidermal growth factor, insulin, and interleukin-6 were able to induce the [3H]thymidine incorporation into DNA in these two cell lines. GRM02, with a doubling time of 36±2 h and a hypertriploid modal chromosome number, produced progesterone and activinlike and inhibinlike material. Follicle-stimulating hormone and luteinizing hormone were able to enhance the secretion of progesterone. For this cell line, only insulin was shown to induce [3H]thymidine incorporation into DNA.
Similar content being viewed by others
References
Adashi, E. Y.; Resnick, C. E.; D’Ercole, A. J., et al. Insulin-like growth factors as intra-ovarian regulators of granulosa cell growth and function. Endocrinol. Rev. 6:400–420; 1985.
Amsterdam, A.; Zauberman, A.; Meir, G., et al. Cotransfection of granulosa cells with simian virus 40 and HaRAS oncogene generate stable lines capable of induced steroidogenesis. Proc. Natl. Acad. Sci. USA 85:7582–7586; 1988.
Amsterdam, A.; Rotmensch, S. Structure-function relationships during granulosa cell differentiation. Endocrinol. Rev. 8:309–337; 1987.
Ben Ze’ev, A.; Amsterdam, A. Regulation of cytoskeletal proteins involved in cell contact formation during differentiation of granulosa cells on extracellular matrix. Proc. Natl. Acad. Sci. USA 83:2894–2898; 1986.
Bendell, J. J.; Dorrington, J. H. Epidermal growth factor influences growth and differentiation of rat granulosa cells. Endocrinology 127:533–540; 1990.
Bernath, V. A.; Muro, A. F.; Vitullo, A. D., et al. Cyclic AMP inhibits fibronectin gene expression in a newly developed granulosa cell line by a mechanism that suppresses cAMP-responsive-dependent transcriptional activation. J. Biol. Chem. 265:18219–18226; 1990.
Birren, S. J.; Anderson, D. J. A vMYC-immortalized sympathoadrenal progenitor cell line in which neuronal differentiation is initiated by FGF but not NGF. Neuron 4:189–201; 1990.
Burowiec, J. A.; Dean, F. B.; Bullock, P. A., et al. Binding and unwinding—how T antigen engages the SV40 origin of DNA replication. Cell 60:181–184; 1990.
Channing, C. P.; Ledwitz-Rigby, F. Methods for assessing hormone-mediated differentiation of ovarian cells in culture and in short term incubations. Methods Enzymol. 39:183–230; 1975.
Chapekar, T. N.; Malik, A. K. The AIMS/GRXVIII cell line: spontaneous transformation of hormonally induced primary cells derived from goat ovarian granulosa. Pathobiology 59:345–350; 1991.
Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156; 1987.
Chou, J. Y. Differentiated mammalian cell lines immortalized by temperature-sensitive tumor viruses. Mol. Endocrinol. 3:1511–1514; 1989.
Cone, J. L.; Brown, D. R.; Delarco, J. E. An improved method of purification of transforming growth factor, type beta from platelets. Anal. Biochem. 168:71–74; 1988.
De Winter, J. P.; Timmerman, M. A.; Vanderstichele, H. M. J., et al. Testicular Leydig cells in vitro secrete only inhibin α-subunits, whereas Leydig cell tumors can secrete bioactive inhibin. Mol. Cell. Endocrinol. 83:105–115; 1992.
Dorrington, J. H.; Bendell, J. J.; Chuma, A., et al. Actions of growth factors in the follicle. J. Steroid Biochem. 27:1–3; 1987.
Dorrington, J.; Chuma, A. V.; Bendell, J. J. Transforming growth factor β and follicle-stimulating hormone promote rat granulosa cell proliferation, Endocrinology 123:353–359; 1988.
Dupont, A. G.; Gerlo, E.; Van der Niepen, P., et al. Renal and pharmacodynamic effects of torasemide and furasemide in normal man. Arzneimittel-Forschung 38:172–175; 1988.
Eckhart, W. Oncogenes of DNA tumor viruses: papovaviruses. In: Weinberg, R. A., ed. Oncogenes and the molecular origins of cancer. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989:223–238.
Erisman, M. D.; Astrin, S. M. The myc oncogene. In: Reddy, E. P.; Skalka, A. M.; Curran, T., eds. The oncogene handbook. Amsterdam, Elsevier Science Publishers B.V. (Biomedical Div.); 1988:341–379.
Felger, P. H.; Gadek, T. R.; Holm, M., et al. Lipofection: a highly efficient, lipid mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA 84:7413–7417; 1987.
Fitz, T. A.; Wah, R. M.; Schmidt, W. A., et al. Physiologic characterization of transformed and cloned rat granulosa cells. Biol. Reprod. 40:250–258; 1989.
Geisthovel, F.; Moretti-Rojas, I.; Rojas, F. J., et al. Insulin-like growth factors and thecal-granulosa-cell function. Human Reprod. 5:785–799; 1990.
Gillies, R. J.; Didier, N.; Denton, M. Determination of cell number in monolayer cultures. Anal. Biochem. 159:109–113; 1986.
Hillensjö, T.; Magnusson, C.; Svensson, U., et al. Effect of luteinizing hormone and follicle-stimulating hormone on progesterone synthesis by cultured rat cumulus cells. Endocrinology 108:1920–1924; 1981.
Hsueh, A. J. W.; Adashi, E. Y.; Jones, P. B. C., et al. Hormonal regulation of the differentiation of cultured ovarian granulosa cells. Endocrinol. Rev. 5:76–126; 1984.
Hunter, T. Cooperation between oncogenes. Cell 64:249–270; 1991.
Hurlin, P. J.; Maher, V. M.; McCormick, J. J. Malignant transformation of human fibroblasts by expression of a transfected T24 HRAS oncogene. Proc. Natl. Acad. Sci. USA 86:187–191; 1989.
Jones, P. B. C.; Welsh, T. H., Jr.; Hsueh, A. J. W. Regulation of ovarian progestin production by EGF in cultured ovarian cells. J. Biol. Chem. 257:11268–11273; 1982.
Laekeman, G. M.; Vergote, I. B.; Keersmaekers, G. M., et al. Prostacyclin and thromboxane in benign and malignant breast tumours. Br. J. Cancer 54:431–437; 1986.
Langhout, D. J.; Spicer, L. J.; Geisert, R. D. Development of a culture system for bovine granulosa cells: effects of growth hormone, estradiol and gonadotropins on cell proliferation, steroidogenesis, and protein synthesis. J. Anim. Sci. 69:3321–3334; 1991.
Lee, V. W. K.; Colvin, N.; Quigg, H., et al. A rapid, sensitive and reliable assay for inhibin bioactivity. Aust. J. Biol. Sci. 40:105–113; 1987.
MacPherson, I. Soft agar technique. In: Kruse, P. F.; Patterson, M. K., eds. Tissue culture methods and applications. New York, Academic Press; 1973:276–280.
May, J. V.; Frost, J. P.; Schomberg, D. W. Differential effects of epidermal growth factor, somatomedin C: insulin-like growth factor I, and transforming growth factor β on porcine granulosa cell deoxyribonucleic acid synthesis and cell proliferation. Endocrinology 123:168–179; 1988.
Michalovitz, D.; Fischer-Fantuzzi, L.; Vesco, C., et al. Activated Ha-ras can cooperate with defective simian virus 40 in the transformation of nonestablished rat embryo fibroblasts. J. Virol. 61:2648–2654; 1987.
Mondschein, J. S.; Canning, S. F.; Hammond, J. M. Effects of transforming growth factor β on the production of immunoreactive insulin-like growth factor I and progesterone and on [3H]thymidine incorporation in porcine granulosa cell cultures. Endocrinology 123:1970–1976; 1988.
Mondschein, J. S.; Canning, S. F.; Miller, D. Q., et al. Insulin-like growth factors (IGFs) as autocrine/paracrine regulators of granulosa cell differentiation and growth: studies with neutralizing antibody to IGF-I. Biol. Reprod. 46:79–85; 1989.
Moses, H. L.; Yang, E. Y.; Pietenpol, J. A. TGF-β stimulation and inhibition of cell proliferation: new mechanistic insights. Cell 63:245–247; 1990.
Poretzky, L.; Kalin, M. F. The gonadotropic function of insulin. Endocrinol. Rev. 8:132–141; 1987.
Rao, I. M.; Mills, T. M.; Anderson, E., et al. Heterogeneity in granulosa cells of developing rat follicles. Anat. Rec. 229:177–185; 1991.
Rodgers, R. J.; Stuchbery, S. J.; Findlay, J. K. Inhibin mRNAs in ovine and bovine ovarian follicles and corpora lutea throughout the estrous and gestation. Mol. Cell. Endocrinol. 62:95–101; 1989.
Sambrook, J.; Fritsch, E. F.; Maniatis, T. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989.
Schwab, M. The myc-box oncogenes. In: Reddy, E. P.; Skalka, A. M.; Curran, T., eds. The oncogene handbook. Amsterdam, Elsevier Science Publishers B.V. (Biomedical Division); 1988:381–391.
Shay, J. W.; Wright, W. E.; Werbin, H. Defining the molecular mechanisms of human cell immortalization. Biochim. Biophys. Acta 1072:1–7; 1991.
Stein, L. S.; Stoica, G.; Tilley, R., et al. Rat ovarian granulosa cell culture: a model system for the study of cell-cell communication during multistep transformation. Cancer Res. 51:696–706; 1991.
Suh, B.; Amsterdam, A. Establishment of highly steroidogenic granulosa cell lines by cotransfection with SV40 and Ha-ras oncogene: induction of steroidogenesis by cyclic adenosine 3′-5′-monophosphate and its suppression by phorbol ester. Endocrinology 127:2489–2500; 1990.
Van der Hurk, R.; Dijkstra, G. An immunohistochemical study of bovine antral follicles, with special attention to non-atretic follicles with and without atypical granulosa cells. Vet Q. 14:148–151; 1992.
Vergote, I. B.; Laekeman, G. M.; Keersmaekers, G. M., et al. Prostaglandin F2α in benign and malignant breast tumours. Br. J. Cancer 51:827–836; 1985.
Verhoeven, G.; Dierckx, P.; De Moor, P. Stimulatory effect of neurotransmitters on the aromatization of testosterone by Sertoli cell-enriched cultures. Mol. Cell. Endocrinol. 13:241–253; 1979.
Verhoeven, G.; Koninckx, P.; De Moor, P. Androgen and progesterone production in cultured interstitial cells derived from immature rat testes. J. Steroid Biochem. 17:319–330; 1982.
Wilkinson, M. RNA isolation: a mini prep method. Nucleic Acid Res. 16:10933; 1988.
Yu, J.; Shao, L.; Lemas, V., et al. Importance of FSH-releasing protein and inhibin in erythrodifferentiation. Nature 330:765–767; 1987.
Zhiwen, Z.; Findlay, J. K.; Carson, R. S., et al. Transforming growth factor β enhances basal and FSH-stimulated inhibin production by rat granulosa cells in vitro. Mol. Cell. Endocrinol. 58:161–166; 1988.
Zilberstein, M.; Chou, J. Y.; Lowe, W. L., et al. Expression of insulin-like growth factor-I and its receptor by SV40 transformed rat granulosa cells. Mol. Endocrinol. 3:1488–1497; 1989.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Briers, T.W., Van De Voorde, A. & Vanderstichele, H. Characterization of immortalized mouse granulosa cell lines. In Vitro Cell Dev Biol - Animal 29, 847–854 (1993). https://doi.org/10.1007/BF02631362
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02631362