Summary
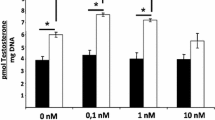
We sought to establish conditions that increased the duration of testosterone production by fully differentiated adult rat Leydig cells in primary culture. A freshly isolated suspension of highly purified adult rat Leydig cells produced 83 ng testosterone/106 Leydig cells·h−1 when incubated in Medium 199 in a 1.5 ml microfuge tube with shaking for 3 h with a maximally stimulating concentration of ovine luteinizing hormone (LH). Unfortunately, adult rat Leydig cells that were allowed to attach only to a plastic culture dish flattened out, and testosterone production diminished rapidly. Leydig cells in Dulbecco's modified Eagles' medium-Ham's F12 (1∶1; vol/vol) containing Cytodex 3 beads pre-equilibrated in culture medium containing fetal bovine serum attached to the beads and remained viable, but produced only 30 ng testosterone/106 Leydig cells·h−1 when incubated for 24 h with similar stimulation. Leydig cells similarly cultured and maximally stimulated with LH, responded to bovine lipoproteins (<1.222 g/ml) producing 105 ng of testosterone/106 Leydig cells·h−1 when incubated with 1 mg/ml bovine lipoprotein. Therefore, lipoproteins maintain the steroidogenic capacity of purified adult rat Leydig cells in primary culture for 24 h.
Similar content being viewed by others
References
Anderson, J. M.; Dietschy, J. M. Relative importance of high and low density lipoproteins in the regulation of cholesterol synthesis in the adrenal gland, ovary, and testis of the rat. J. Biol. Chem. 253:9024–9032; 1978.
Benahmed, M.; Saez, J. M. Role of lipoproteins in the regulation of Leydig cell steroidogensis. In: Strauss, J. R.; Menon, K. M. J., eds. Lipoprotein and cholesterol metabolism in steroidogenic tissues. Philadelphia, PA: George F. Stickley Co., 1985:103–110.
Bordy, M. J.; Shaper, J. H.; Ewing, L. L. Trophic influences of luteinizing hormone on steroidogenesis by Percoll-separated rat Leydig cells in culture. In: Catt, K. J.; Dufau, M. L., eds. Hormone action and testicular function, vol. 438. New York: New York Academy of Sciences; 1984:329–345.
Browning, J. Y.; Heindel, J. J.; Grotjen, J. H. E. Primary culture of purified Leydig cells isolated from adult rat testes. Endocrinology 112:543–549; 1983.
Chubb, C.; Ewing, L. L. Steroid secretion byin vitro perfused testes: testosterone biosynthetic pathways. Am. J. Physiol. 237:E231–238; 1979.
Darney, K. J., Jr.; Wing, T.-Y.; Ewing, L. L. Simultaneous measurement of four testicular Δ4-3-ketosteroids by isouratic high-performance liquid chromatography with on-line ultraviolet absorbance detection. J. Chromatography 257:81–90; 1983.
Durand, P.; Cathiard, A. M.; Naaman, E., et al. The influence of plasma lipoproteins on steroidogenesis of cultured ovine fetal and neonatal adrenal cells. J. Steroid Biochem. 26:425–431; 1987.
Hauel, R. J.; Eder, H. A.; Bragdon, J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34:1345–1353; 1955.
Hunter, M. G.; Cooke, B. A. The functional activity of adult mouse Leydig cells in monolayer culture: effect of lipoproteins, pregnenolone and choleratoxin. Mol. Cell. Endocrinol. 39:217–228; 1985.
Klinefelter, G. R.; Hall, P. F.; Ewing, L. L. Effect of luteinizing hormone deprivationin situ on steroidogenesis of rat Leydig cells purified by a multistep procedure. Biol. Reprod. 36:769–783; 1987.
Kovanen, P. T.; Faust, J. R.; Brown, M. S., et al. Low density lipoprotein receptors in bovine adrenal cortex. I. Receptor-mediated uptake of low density lipoprotein and utilization of its cholesterol for steroid synthesis in cultured adrenocortical cells. Endocrinology 104:599–609; 1979.
Lowry, O. H.; Rosenbrough, N. J.; Farr, A. L., et al. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275; 1951.
Mather, J. P.; Saez, J. M.; Haour, F. Primary culture of Leydig cells from rat, mouse, and pig: advantages of porcine cells for the study of gonadotropin regulation of Leydig cell function. Steroids 38:35–39; 1981.
Rommerts, F. F. G.; vanRosenburg, M. J. A.; Lindh, L. M., et al. The effect of short-term culture and perifusion on LH-dependent steroidogenesis in isolated rat Leydig cells. J. Reprod. Fert. 65:289–297; 1982.
Sharpe, R. M.; Cooper, I. Relationship between the exposure of Leydig cells to factor(s) present in testicular interstitial fluid and changes in their capacity to secrete testosterone during culture of after hCG-induced desensitization. Mol. Cell. Endocrinol. 51:105–114; 1987.
Quinn, P. G.; Dombrovsky, L. J.; Chen, Y. I., et al. Serum lipoproteins increase testosterone production in hCG-desensitized Leydig cells. Endocrinology 109:1790–1792; 1981.
Quinn, P. G.; Georgiou, M.; Payne, A. H. Differences in hydroxysterol metabolism between rat and mouse Leydig cells. In: Strauss, J. F.; Menon, K. M. J., eds. Lipoprotein and cholesterol metabolism in steroidogenic tissues. Philadelphia, PA: George F. Stickley Co.; 1985:243–246.
Author information
Authors and Affiliations
Additional information
This research was supported in part by the National Institutes of Health (grant HD-07204), The Population Center (grant HD-06268), and EPA cooperative agreement (CR81-2765), an NSF equipment grant, and a Mellon Foundation Postdoctoral Fellowship for Gary Klinefelter. Although the research described herein has been funded in part by the U.S. Environmental Protection Agency through cooperative agreement (CR81-2765) to the Division of Reproductive Biology at Johns Hopkins University, it has not been subjected to the agency's peer and policy review, and therefore, does not necessarily reflect the views of the agency and no official endorsement should be inferred.
Rights and permissions
About this article
Cite this article
Klinefelter, G.R., Ewing, L.L. Optimizing testosterone production by purified adult rat Leydig cells in vitro. In Vitro Cell Dev Biol 24, 545–549 (1988). https://doi.org/10.1007/BF02629089
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02629089