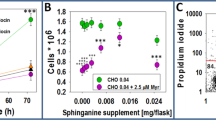
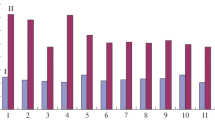
Summary
Plasma membranes isolated from HeLa cells cultivated in suspension cultures supplemented with 3.5% fetal bovine serum or 2% of the commercially available serum substitute Ultroser G contained the same amounts of protein, cholesterol, and phosphate on a cellular basis. Minor differences in the plasma membrane fatty acid composition were seen, with the most pronounced alteration observed for palmitic acid, which amounted to 27 and 20% in fetal bovine serum- and Ultroser G-supplemented cells, respectively. Plasma membranes from cells growth with Ultroser G contained almost twice as much phosphatidylethanolamine and displayed two thirds of the phosphatidylcholine content, compared to plasma membranes obtained from fetal bovine serum supplemented cells. The former membranes also showed a 3 times higher specific [3H]acetate labeling of cholesterol, indicating a higherde novo synthesis of cholesterol. Both quantitative and qualitative alterations were revealed among the plasma membrane polypeptides when these were subjected to immuno- and lectin blottings. Fluorescence anisotropy measurements at different temperatures produced similar results irrespective of the growth medium supplement when the plasma membrane specific probe 1-(4-trimethylammoniumphenyl)-6-phenyl-1,3,5-hexatriene was used on intact cells. However, the average cellular rigidity was higher for Ultroser G supplemented cells, determined with 1,6-diphenyl-1,3,5-hexatriene as a probe.
Similar content being viewed by others
References
Ames, G. F. Lipids ofSalmonella typhimurium andEscherichia coli: structure and metabolism. J. Bacteriol. 95:833–843; 1968.
Atkinson, P. H.; Summers, D. F. Purification and properties of HeLa cell plasma membranes. J. Biol. Chem. 246:5162–5175; 1971.
Barnes, D.; Sato, G. Methods for growth of cultured cells in serum-free medium. Anal. Biochem. 102:255–270; 1980.
Barnes, D.; Sato, G. Serum-free cell culture: a unifying approach. Cell 22:649–655; 1980.
Bettger, W. J.; Boyce, S. T.; Walthall, B. J., et al. Rapid clonal growth and serial passage of human diploid fibroblasts in a lipid-enriched synthetic medium supplemented with epidermal growth factor, insulin, and dexamethasone. Proc. Natl. Acad. Sci. USA 78:5588–5592; 1981.
Blake, M. S.; Johnston, K. H.; Russell-Jones, G. J., et al. A rapid, sensitive method for detection of alkaline phosphataseconjugated anti-antibody on Western blots. Anal. Biochem. 136:175–179; 1984.
Bligh, E. G.; Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911–917; 1959.
Bottenstein, J. E.; Sato, G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc. Natl. Acad. Sci. USA 76:514–517; 1979.
Brown, M. S.; Goldstein, J. L. Receptor-mediated endocytosis: insight from the lipoprotein receptor system. Proc. Natl. Acad. Sci. USA 76:3330–3337; 1979.
Brunette, D. M.; Till, J. E. A rapid method for the isolation of L-cell surface membranes using an aqueous two-phase polymer system. J. Membr. Biol. 5:215–224; 1971.
Burns, C. P.; Wei, S-P. L.; Spector, A. A. Fatty acid metabolism in L1210 murine leukemia cells: differences in modification of fatty acids incorporated into various lipids. Lipids 13:666–672; 1978.
Esko, J. D.; Gilmore, J. R.; Glaser, M. Use of a fluorescent probe to determine the viscosity of LM cell membranes with altered phospholipid composition. Biochemistry 16:1881–1890; 1977.
Esko, J. D.; Matsuoka, K. Y. Biosynthesis of phosphatidylcholine from serum phospholipids in chinese hamster ovary cells deprived of choline. J. Biol. Chem. 258:3051–3057; 1983.
Esko, J. D.; Raetz, C. R. H. Synthesis of phospholipids in animal cells. In: Boyer, P. D., ed. The enzymes, vol. 16. Lipid enzymology. New York: Academic Press; 1983:207–253.
Everitt, E.; Svensson, U.; Wohlfart, C., et al. Requirements for initial adenovirus uncoating and internalization. In: Crowell, R. L.; Lonberg-Holm, K. eds. Virus attachment and entry into cells. Washington, DC: American Society for Microbiology; 1986:196–204.
Freter, C. E.; Ladenson, R. C.; Silbert,D. F. Membrane phospholipid alterations in response to sterol depletion of LM cells. J. Biol. Chem. 254:6909–6916; 1979.
Hartree, E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal. Biochem. 48:422–427; 1972.
Hutchings, S. E.; Sato, G. H. Growth and maintenance of HeLa cells in serum-free medium supplemented with hormones. Proc. Natl. Acad. Sci. USA 75:901–904; 1978.
Illingworth, D. R.; Portman, O. W.; Robertson, A. L., Jr., et al. The exchange of phospholipids between plasma lipoproteins and rapidly dividing human cells grown in tissue culture. Biochim. Biophys. Acta306:422–436; 1973.
Maizel, J. V., Jr. Polyacrylamide gel electrophoresis of viral proteins. In: Maramorosch, K.; Koprowski, H., eds. Methods in virology, vol. V. New York/London: Academic Press Inc.; 1971:179–246.
McClare, C. W. F. An accurate and convenient organic phosphorus assay. Anal. Biochem 39:527–530; 1971.
McGee, R., Jr. Membrane fatty acid modification of the neuroblastoma X glioma hybrid, NG108-15. Biochim. Biophys. Acta 663:314–328; 1981.
Morrison, R. S.; de Vellis, J. Growth of purified astrocytes in chemically defined medium. Proc. Natl. Acad. Sci. USA 78:7205–7209; 1981.
Myara, I.; Polini, G.; Scotto, J., et al. The use of Ultroser G as a serum substitute in the culture of human skin fibroblasts. Biol. Cell 57:243–248; 1986.
Peterson, J. A.; Rubin, H. The exchange of phospholipids between cultured chick embryo fibroblasts and their growth medium. Exp. Cell Res. 58:365–378; 1969.
Portman, O. W.; Illingworth, D. R. Lysolecithin binding to human and squirred monkey plasma and tissue components. Biochim. Biophys. Acta. 326:34–42; 1973.
Prendergast, F. G.; Haugland, R. P.; Callahan, P. J. 1-[4-(Trimethylamino)phenyl]-6-phenylhexa-1,3,5-triene: synthesis, fluorescence properties, and use as a fluorescence probe of lipid bilayers. Biochemistry 20:7333–7338; 1981.
Product Information, Ultroser G, No. 202908, Villeneuve-la-Gavenne, France: Réactifs IBF.
Roelofsen, B.; Zwaal, R. F. A. The use of phospholipases in the determination of asymmetric phospholipid distribution in membranes. In: Korn, E. D., ed. Methods in membrane biology, vol. 7. New York: Plenum Press; 1976:147–177.
Ronot, X.; Sene, C.; Boschetti, E., et al. Culture of chondrocytes in medium supplemented with fetal calf serum or a serum substitute.Ultroser G. Biol. Cell 51:307–314; 1984.
Shinitzky, M.; Barenholz, Y.Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim. Biophys. Acta 515:367–394; 1978.
Spector, A. A.; Kiser, R. E.; Denning, G. M., et al. Modification of the fatty acid composition of cultured human fibroblasts. J. Lipid Res.20:536–547; 1979.
Spector, A. A.; Mathur, S. N.; Kaduce, T. L., et al. Lipid nutrition and metabolism of cultured mammalian cells. Prog. Lipid Res. 19:155–186; 1981.
Stubbs, C. D.; Smith, A. D. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim. Biophys. Acta 779:89–137; 1984.
Sundler, R.; Åkesson, B. Regulation of phospholipid biosynthesis in isolated rat hepatocytes. J. Biol. Chem. 250:3359–3367; 1975.
Svensson, U.; Persson, R.; Everitt, E. Virus-receptor interaction in the adenovirus system. I. Identification of virion attachment proteins of the HeLa cell plasma membrane. J. Virol. 38:70–81; 1981.
Switzer, S.; Eder, H. A. Transport of lysolecithin by albumin in human and rat plasma. J. Lipid Res. 6:506–511; 1965.
Taub, M.; Chuman, L.; Saier, M. H., Jr., et al. Growth of Madin-Darby canine kidney epithelial cell (MDCK) line in hormone-supplemented, serum-free medium. Proc. Natl. Acad. Sci. USA 76:3338–3342; 1979.
Tijburg, L. B. M.; Geelen, M. J. H.; Van Golde, L. M. G. Biosynthesis of phosphatidylethanolamine via the CDP-ethanolamine route is an important pathway in isolated rat hepatocytes. Biochem. Biophys. Res. Commun. 160:1275–1280; 1989.
Vance, D. E. Phospholipid metabolism in eucaryotes. In: Vance, D. E.; Vance, J. E. eds. Biochemistry of lipids and membranes. Menlo Park, CA: The Benjamin/Cummings Publishing Company, Inc.; 1985:242–270.
Wu, R.; Sato, G. H. Replacement of serum in cell culture by hormones: a study of hormonal regulation of cell growth and specific gene expression. J. Toxicol. Environ. Health 4:427–448; 1978.
Yeoh, G.; Douglas, A.; Brighton, V. Long-term culture of fetal rat hepatocytes in media supplemented with fetal calf serum, Ultroser SF or Ultroser G. Biol. Cell 58:53–64; 1986.
Author information
Authors and Affiliations
Additional information
This investigation was supported by grants from the Swedish Natural Science Research Council, Anders Otto Swärds Stiftelse, Stockholm, Crafoordska Stiftelsen, Lund and Kungl. Fysiografiska Sällskapet, Lund.
Rights and permissions
About this article
Cite this article
Blixt, Y., Valeur, A. & Everitt, E. Cultivation of HeLa cells with fetal bovine serum or ultroser G: Effects on the plasma membrane constitution. In Vitro Cell Dev Biol 26, 691–700 (1990). https://doi.org/10.1007/BF02624425
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02624425