Summary
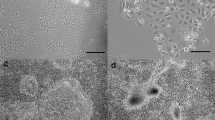
A method is described for culturing human mammary epithelial cells in primary culture and allowing more than 50 generations and a 1000-fold increase from starting inocula without need of enzymatic transfers. Organoids dissociated from breast tissue are plated in medium containing 1.05 mM Ca++ to effect attachment and growth to monolayer density. Medium is then switched to one containing 0.06 mM Ca++ to overcome “renewal inhibition” and to stimulate growth. In low Ca++ media, primary cultures become a long-term, continuous source of free-floating viable cells free of fibroblasts. A fundamental requirement for extended growth in primary culture is maintaining calcium levels at approximately 0.06 mM. Above 0.06 mM Ca++, cells divide only 3 to 4 times in primary cultures before terminal differentiation occurs. At 0.06 mM Ca++, cells continue to divide for periods of time determined partly by feeding schedule, but up to 6 mo. and 50 generations of (linear) growth. Cells released from monolayer were greater than 90% viable and yielded 105 cells/cm2 of attached cells every 72 h. Free-floating single cells readily replated and cloned, when transferred, without need of trypsin for dissociation. Long-term free-floating cells were typical mammary epithelium: (a) they formed domes and exhibited renewal inhibition, (b) they produced ductlike formations in collagen gels, (c) they contained epithelium-specific keratin filaments, and (d) they were diploid.
Similar content being viewed by others
References
Brennan, J. K.; Mansky, J.; Roberts, G., et al. Improved methods for reducing calcium and magnesium concentrations in tissue culture medium: Application to studies of lymphoblast proliferation in vitro. In Vitro 11:354–360; 1975.
Butler, W. B. Preparing nuclei from cells in monolayer cultures suitable for counting and for following synchronized cells through the cell cycle. Anal. Biochem. 141:70–73; 1984.
Cali, J. P.; Mandel, J.; Moore, L., et al. A referee method for the determination of calcium in serum. U. S. National Bureau of Standards Spec. Publ. 260-36. Washington, DC: U. S. Government Printing Office; 1972.
Cristofalo, V. J.; Wallace, J. M.; Rosner, B. A. Glucocorticoid enhancement of proliferative activity in WI-38 cells. In: Sato, G. H.; Ross, R., eds. Hormones and cell culture, book B. New York: Cold Spring Harbor Laboratory; 1979:875–887.
Hammond, S. L.; Ham, R. G.; Stampfer, M. R. Serum-free growth of human mammary epithelial cells: Rapid clonal growth in defined medium and extended serial passage with pituitary extract. Proc. Natl. Acad. Sci. USA 81:5435–5439; 1984.
Lazarides, E. Intermediate filaments as mechanical integrators of cellular space. Nature 283:249–256; 1980.
Lechner, J. F.; Haugen, A.; Autrup, H., et al. Clonal growth of epithelial cells from normal adult human bronchus. Cancer Res. 41:2294–2304; 1981.
McGrath, C. M.; Soule, H. D. Renewal inhibition of human mammary cell growth in vitro: Cortisol and the recruitment of cells to terminal differentiation. J. Cell Physiol. 116:385–396; 1983.
McGrath, C. M.; Soule, H. D. Calcium regulation of normal human mammary epithelial cell growth in culture. In Vitro 20:652–662; 1984.
Soule, H. D.; Vazquez, J.; Long, A., et al. A human cell line from a pleural effusion derived from a breast carcinoma. J. Natl. Cancer Inst. 51:1409–1416; 1973.
Stampfer, M. R.; Hallowes, R. C.; Hackett, A. J. Growth of normal human mammary cells in culture. In Vitro 615:415–425; 1980.
Stampfer, M. R. Cholera toxin stimulation of human mammary epithelial cells in culture. In Vitro 18:531–537; 1982.
Stampfer, M. R.; Hackett, A. J.; Smith, H. S., et al. Growth of human mammary epithelium in culture and expression of tumor-specific properties. In: Sirbasku, B. A.; Pardee, A. B.; Sato, G. H., eds. Growth of cells in hormonally defined medium. Vol. 9. New York: Cold Spring Harbor Laboratory; 1982:819–829.
Stoner, G. D.; Babcock, M. S. Influence of growth factors on proliferation of normal and chemically transformed rat esophageal epithelial cells. Proc. Am. Assoc. Cancer Res. 23:42; 1982.
Yang, J.; Richards, J.; Bowman, P., et al. Sustained growth and three-dimensional organization of primary mammary tumor epithelial cells embedded in collagen gels. Proc. Natl. Acad. Sci. USA 76:3401–3405; 1979.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Soule, H.D., McGrath, C.M. A simplified method for passage and long-term growth of human mammary epithelial cells. In Vitro Cell Dev Biol 22, 6–12 (1986). https://doi.org/10.1007/BF02623435
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02623435