Abstract
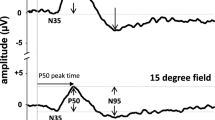
The pattern electroretinogram was recorded to checkerboard stimuli with a wide range of check sizes and two stimulus field sizes. Check sizes ranged from 0.25° to 7° (field size, 16°×14°) and 0.25° to 15° (field size, 32°×27°) in 14 and seven subjects, respectively. Reversal rate was 4.5/s. For minimal intrusion of blink artifacts the interrupted stimulation technique was employed. The P50 and N95 components of the pattern electroretinogram were evaluated separately. With both stimulus field sizes amplitude of P50 and N95 was maximal between 0.75° and 1°. With smaller check sizes the amplitude dropped monotonically. With larger check sizes field size played a role: with the 16°×14° field, P50 gradually dropped to 89% from 1° to 7°, which was paralleled by N95 only up to 7°, where N95 dropped to 81% (p<0.05). With the 32°×27° field, there was no significant difference in size dependency between P50 and N95 for large checks, both components staying constant from 1° to 15° We conclude that there is only minor large-check attenuation of the pattern electroretinogram, especially with a large field. The apparent field-size dependency may explain previous discrepancies in the literature.
Similar content being viewed by others
Abbreviations
- LSFA:
-
Iow-spatial frequency attenuation
References
Groneberg A, Teping C. Topodiagnostik von Sehstörungen durch Ableitung retinaler und kortikaler Antworten auf Umkehr-Kontrastmuster. Ber Dtsch Ophthalmol Ges 1980; 77: 409–15.
Maffei L, Fiorentini A. Electroretinographic responses to alternating gratings before and after section of the optic nerve. Science 1981; 211: 953–4.
Harrison JM, O'Connor PS, Young RSL, Kincaid M, Bentley R. The pattern ERG in man following surgical resection of the optic nerve. Invest Ophthalmol Vis Sci 1987; 28: 492–9.
Bach M, Gerling J, Geiger K. Optic atrophy reduces the pattern-electroretinogram for both fine and coarse stimulus patterns. Clin Vision Sci 1992; 7: 327–33.
Sieving PA, Steinberg RH. Proximal retinal contributions to the intraretinal 8-Hz pattern ERG of cat. J Neurophysiol 1987; 57: 104–20.
Baker CL, Hess RF, Olsen BT, Zrenner E. Current source density analysis of linear and non-linear components of the primate electroretinogram. J Physiol 1988; 407: 155–76.
Enroth-Cugell C, Robson JG. Functional characteristics and diversity of cat retinal ganglion cells: basic characteristics and quantitative description. Invest Ophthalmol Vis Sci 1984; 25: 250–67.
Maffei L. Electroretinographic and visual cortical potentials in response to alternating gratings. Ann NY Acad Sci 1982; 388: 1–10.
Riemslag FCC, Ringo JL, Spekreijse H, Verduyn Lunel H. The distinction between luminance and spatial contrast components in the pattern ERG. Doc Ophthalmol Proc Ser 1983; 37: 255–64.
Korth M. Pattern-evoked responses and luminance-evoked responses in the human electroretinogram. J Physiol 1983; 337: 451–69.
Hess RF, Baker CL. Human pattern-evoked electroretinogram. J Neurophysiol 1984; 51: 939–51.
Korth M, Rix R. Changes in spatial selectivity of pattern-ERG components with stimulus contrast. Graefes Arch Clin Exp Ophthalmol 1985; 223: 23–8.
Bach M, Speidel-Fiaux A. Pattern electroretinogram in glaucoma and ocular hypertension. Doc Ophthalmol 1989; 73: 173–81.
Korth M. Human fast retinal potentials and the spatial properties of a visual stimulus. Vision Res 1981; 21: 627–30.
Arden GB, Vaegan, Hogg CR. Clinical and experimental evidence that the pattern electroretinogram (PERG) is generated in more proximal retinal layers than the focal ERG (FERG). Ann NY Acad Sci 1982; 388: 580–607.
Kirkham TH, Coupland SG. Pattern ERGs and check size.: absence of spatial frequency tuning. Curr Eye Res 1982; 2: 511–21.
Trick GL, Wintermeyer DH. Spatial and temporal frequency tuning of pattern-reversal retinal potentials. Invest Ophthalmol Vis Sci 1982; 23: 774–9.
Vaegan, Arden GB, Hogg CR. Properties of normal electroretinograms evoked by parterned stimuli in man. Doc Ophthalmol Proc Ser 1982; 31: 111–29.
Sokol S, Jones K, Nadler D. Comparison of spatial properties of human retina and cortex as measured by simultaneously recorded pattern ERGs and VEPs. Vision Res 1983; 23: 723–7.
Porciatti V, Francesconi W, Bagnoli P. The pigeon pattern electroretinogram is not affected by massive loss of cell bodies in the ganglion layer induced by chronic section of the optic nerve. Doc Ophthalmol 1985; 61: 41–7.
Tobimatsu S, Celesia GG, Cone S, Gujrati M. Electroretinograms to checkerboard pattern reversal in cats: physiological characteristics and effect of retrograde degeneration of ganglion cells. Electroencephalog Clin Neurol 1989; 73: 341–52.
Holländer H, Bisti S, Maffei L, Hebel R. Electroretinographic responses and retrograde: changes after intracranial optic nerve section: a quantitative analysis in the cat. Exp Brain Res 1984; 55: 483–493.
Berninger T, Schuurmans RP. Spatial tuning of the pattern ERG across temporal frequency. Doc Ophthalmol 1985; 61: 17–25.
Holder GE. Pattern ERG abnormalities in anterior visual pathway disease. Electroencephalog Clin Neurophysiol 1985; 61: S 135.
Holder GE. Significance of abnormal pattern electroretinography in anterior visual pathway dysfunction. Br J Ophthalmol 1987; 71: 166–71.
Ryan S, Arden GB. Electrophysiological discrimination between retinal and optic nerve disorders. Doc Ophthalmol 1988; 68: 247–55.
Bach M, Hiss P, Röver J. Check-size specific changes of pattern electroretinogram in patients with early open-angle glaucoma. Doc Ophthalmol 1988; 69: 315–22.
Bach M, Gerling J. Retinal and cortical activity in human subjects during color flicker fusion. Vision Res 1992; 32: 1219–23.
Holder GE. The incidence of abnormal pattern electroretinography in optic nerve demyelination. Electroencephalog Clin Neurophysiol 1991; 78: 18–26.
Holder GE. Pattern electroretinography in the evaluation of glaucoma and in optic nerve function. In: Heckenlively JR, Arden GB (eds.) Principles and practice of clinical electrophysiology of vision. St. Louis: Mosby-Year Book, 1991; 549–56.
Mauguiere F, Holder GE, Luxon LM, Pottinger R. Evoked potentials: abnormal wave-forms and diagnostic yield of evoked potentials. In: Osselton J, Binnie CD, Cooper R, Fowler CJ, Mauguiere F, Prior PF, (eds.) Clinical neurophysiology: EMG, nerve conduction and evoked potentials. Oxford: Butterworth-Heine 1995: 431–81.
Bach M. The Freiburg Visual Acuity Test—Automatic measurement of visual acuity. Optometry Vision Sci. 1996; 73: 49–53.
Pelli DG, Zhang L. Accurate control of contrast on microcomputer displays. Vision Res 1991; 31: 1337–50.
Holder GE. Recording the pattern electroretinogram with the Arden gold foil electrode. J Electrophysiol Technol 1988; 14: 183–90.
Odom JV, Holder GE, Feghali JG, Cavender S. Pattern electroretinogram intrasession reliability: a two center comparison. Clin Vis Sci 1992; 7: 263–282.
Drasdo N, Thompson DA, Thompson CM, Edwards L. Complementary components and local variations of the pattern electroretinogram. Invest Ophthalmol Vis Sci 1987; 28: 158–62.
van den Berg TJTP, Boltjes B. The point-spread function of the eye from 0° to 100° and the pattern electroretinogram. Doc Ophthalmol 1988; 67: 347–54.
Marmor M, Holder GE, Porciatti V, Trick GL Zrenner E. ISCEV PERG guidelines. Doc Ophthalml. In press.
Vaegan, Arden GB. Effect of pattern luminance profile on the pattern ERG in man and pigeon. Vision Res 1987; 27: 883–92.
Vaegan, Arden GB. Electroretinograms evoked in man by local uniform or unpatterned stimulation. J Physiol 1983; 341: 85–104.
Kelly DHC. Spatial frequency selectivity in the retina. Vision Res 1975; 15: 665–72.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bach, M., Holder, G.E. Check size tuning of the pattern electroretingoram: a reappraisal. Doc Ophthalmol 92, 193–202 (1996). https://doi.org/10.1007/BF02583290
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02583290