Abstract
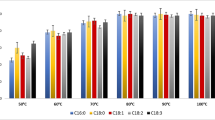
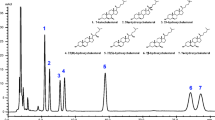
The successive steps of an integrated analytical procedure aimed at the accurate determination of butterfat fatty acid composition, includingtrans-18:1 acid content and profile, have been carefully checked. This sequential procedure includes: dispersion of a portion of butter in hexane/isopropanol (2:1, vol/vol) with anhydrous Na2SO4, filtration of aliquots of the suspension through a microfiltration unit, subsequent preparation of fatty acid isopropyl esters (FAIPE) with H2SO4 as a catalyst, and analysis of total FAIPE by capillary gas-liquid chromatography (GLC). Isolation oftrans-18:1 isomers was by silver-ion thin-layer chromatography (Ag-TLC), followed by extraction from the gel of combined saturated andtrans-monoenoic acids with a biphasic solvent system. Analysis of these fractions by GLC allows the accurate quantitation oftrans-18:1 acids with saturated acids (14:0, 16:0, and 18:0) as internal standards. A partial insight in the distribution oftrans-18:1 isomers can be obtained by GLC on a CP Sil 88 capillary column (Chrompack, Middelburg, The Netherlands). All steps of the procedure are quite reproducible, part of the coefficients of variation (generally less than 3%, mainly limited to butyric and stearic acids) being associated with GLC analysis (injection, integration of peaks) and, to a lesser extent, to FAIPE preparation. FAIPE appear to be of greater practical interest than any other fatty acid esters, including fatty acid methyl esters (FAME), for the quantitation of short-chain fatty acids, because peak area percentages, calculated by the integrator coupled to the flame-ionization detector, are almost equal (theoretically and experimentally) to fatty acid weight percentages and do not require correction factors. With this set of procedures, we have followed in detail the seasonal variations of fatty acids in butterfat, with sixty commercial samples of French butter collected at five different periods of the year. Important variations occur around mid-April, when cows are shifted from forage and concentrates during winters spent in their stalls to fresh grass in pastures. At this period, there is a decrease of 4:0–14:0 acids and of 16:0 (−2 and −6%, respectively), while 18:0 andcis- plustrans-18:1 acids rise suddenly (2 and 5%, respectively). These modifications then progressively disappear until late fall or early winter. Other variations are of minor quantitative importance. Although influenced by the season, the content of 18:2n-6 acid lies in the narrow range of 1.2–1.5%.Trans-18:1 acids, quantitated by GLC after Ag-TLC fractionation, are at their highest level in May–June (4.3% of total fatty acids), and at their lowest level between January and the end of March (2.4%), with a mean annual value of 3.3%. The proportion of vaccenic (trans-11 18:1) acid, relative to totaltrans-18:1 isomers, is higher in spring than in winter, with intermediate decreasing values in summer and fall, which supports the hypothesis that the level of this isomer is linked to the feed of the cattle, and probably to the amount of grass in the feed.
Similar content being viewed by others
References
Wolff, R.L.,J. Am. Oil Chem. Soc. 72:259 (1995).
Christie, W.W.,Prog. Lipid Res. 17:245 (1979).
Bitman, J., and D.L. Wood,J. Dairy Sci. 73:1208 (1990).
Iverson, J.L., and J. Sheppard, Ibid.:1707 (1989).
Gray, I.K.,J. Dairy Res. 40:207 (1973).
Wolff, R.L.,J. Am. Oil Chem. Soc. 71:277 (1994).
Patton, S., R.D. McCarthy, L. Evans, and T.R. Lynn,J. Dairy Sci. 43:1187 (1960).
Jensen, R.G., G.W. Gander, and J. Sampugna, Ibid.:329 (1962).
Boatman, C., D.K. Hotchkiss, and E.G. Hammond, Ibid.:34 (1965).
Hutton, K., R.C. Seeley, and D.G. Armstrong,J. Dairy Res. 36:103 (1969).
Senft, B., and F. Kobasa,Michwissenschaft 25:510 (1970).
Huyghebaert, A., and H. Hendrickx, Ibid.:613 (1971).
Wolff, R.L., and A.F.M. Castera-Rossignol,Rev. Fr. Corps Gras 34:123 (1987).
Wolff, R.L.,Sci. Alim. 13:559 (1993).
Wolff, R.L., and R.J. Fabien,Lait 69:33 (1989).
Badings, H.T., and C. De Jong,J. Chromatogr. 279:493 (1983).
Sampugna, J., R.E. Pitas, and R.G. Jensen,J. Dairy Sci. 49:1462 (1966).
Iverson, J.L., and A.J. Sheppard,Food Chem. 21:223 (1986).
Lund, P., and F. Jensen,Milchwissenschaft 38:193 (1983).
Hay, J.D., and W.R. Morrison,Biochim. Biophys. Acta 202:237 (1970).
Morrison, W.R.,Topics in Lipid Chemistry, Vol. 1, edited by F.D. Gunstone, Logos Press Limited, 1970, p. 52.
Craske, J.D., and C.D. Bannon,J. Am. Oil Chem. Soc. 54:1413 (1987).
Ackman, R.G., and J.C. Sipos, Ibid.:377 (1964).
Ackman, R.G., and J.C. Sipos,J. Chromatogr. 16:298 (1964).
Bannon, C.D., N.T. Hai, N.L. Harper, and K.L. O’Rourke, Ibid.:67 (1982).
Bannon, C.D., J.D. Craske, and A.E. Hilliker,J. Am. Oil Chem. Soc. 62:1501 (1984).
Bannon, C.D., J.D. Craske, and A.E. Hilliker, Ibid.:105 (1986).
Iverson, J.L., and A.J. Sheppard,J. Ass. Off. Anal. Chem. 60:284 (1977).
Traitler, H., U. Richli, A.M. Kappeler, and C. Monnard,6th International Symposium on Capillary Chromatography, Riva del Garda, 1985, p. 568.
Seher, A., and M. Arens,Fat. Sci. Technol. 89:307 (1987).
Parodi, P.W.,Aust. J. Dairy Technol. 25:200 (1970).
Bornaz, S., G. Novak, and M. Parmentier,J. Am. Oil Chem. Soc. 69:1131 (1992).
Gallacier, J.-P., J.-P. Barbier, and S. Kuzdzal-Savoie,Tech. Lait. 993:13 (1984).
Harfoot, C.G.,Prog. Lipid Res. 17:21 (1978).
Kuzdzal-Savoie, S., J. Raymond, W. Kuzdzal, and J. Petit,Ann. Biol. Anim. Biochem. Biophys. 6:351 (1966).
Precht, D.,Z. Ernährungswiss. 34:27 (1995).
Parodi, P.W.,J. Dairy Sci. 59:1870 (1976).
Author information
Authors and Affiliations
About this article
Cite this article
Wolff, R.L., Bayard, C.C. & Fabien, R.J. Evaluation of sequential methods for the determination of butterfat fatty acid composition with emphasis ontrans-18:1 acids. Application to the study of seasonal variations in french butters. J Am Oil Chem Soc 72, 1471–1483 (1995). https://doi.org/10.1007/BF02577840
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02577840